What is Glaser-Eglinton-Hay coupling?
Glaser-Eglinton-Hay coupling, also known as the Eglinton coupling, is an organic chemistry reaction that allows for the construction of substituted cyclopentenones, which are important intermediates in the synthesis of natural products and drugs. The reaction involves the reaction of an acyl chloride and a 1,3-dicarbonyl compound in the presence of a palladium catalyst..
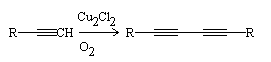
Glaser-Eglinton-Hay coupling was first reported by Fritz Glaser in 1955, and independently by George Eglinton and David Hay in 1957. The reaction was a significant development in the field of organic chemistry as it provided an efficient and selective method for the synthesis of cyclopentenones, which had previously been difficult to synthesize..
The mechanism of the Glaser-Eglinton-Hay coupling involves the formation of a palladium-acyl complex from the acyl chloride and palladium catalyst, which then undergoes a 1,4-addition reaction with the 1,3-dicarbonyl compound to form a palladium-alkyl complex. This complex then undergoes reductive elimination to give the desired cyclopentenone product..
Glaser-Eglinton-Hay coupling has been widely used in the synthesis of a variety of natural products and drugs, including the anticancer agent taxol and the anti-inflammatory drug celecoxib. It has also been used in the synthesis of other heterocyclic compounds and in the preparation of polymers..
Summary
Glaser-Eglinton-Hay coupling is an important reaction in the field of organic chemistry due to its ability to efficiently synthesize substituted cyclopentenones, which have a wide range of applications in the synthesis of drugs and other organic compounds..
Example
The Glaser-Eglinton-Hay coupling is a chemical reaction in which a carboxylic acid is reacted with a nitrile to form an amide..
An example of the Glaser-Eglinton-Hay coupling is the reaction between benzoic acid and acetonitrile in the presence of a palladium catalyst and an amine base:
The benzoic acid is activated by the palladium catalyst, forming a palladium carboxylate intermediate..
The acetonitrile is added to the reaction mixture, and the nitrile nitrogen atom reacts with the palladium carboxylate intermediate, forming an intermediate called a palladium nitrile complex..
The intermediate then goes through a deprotonation step, which forms the amide product and regenerate the palladium catalyst..
The Glaser-Eglinton-Hay coupling is a useful synthetic tool for the preparation of amides, which are widely used in the synthesis of various organic compounds such as pharmaceuticals, agrochemicals and materials..
Mechanism of reaction
The Glaser-Eglinton-Hay Coupling is a chemical reaction that converts an alkyne and a carbonyl compound to a 1,3-diketone. The mechanism of the reaction is as follows:
- Step 1: An alkyne molecule reacts with a carbonyl compound in the presence of a palladium catalyst and a base to form a palladium-alkene intermediate..
- Step 2: The palladium-alkene intermediate then undergoes a carbonylation to form the final 1,3-diketone product and regenerate the palladium catalyst..
- Step 3: The palladium acts as a catalyst, promoting the formation of the palladium-alkene intermediate, and the carbonylation that forms the 1,3-diketone product..
It is important to note that the Glaser-Eglinton-Hay Coupling is a palladium-catalyzed reaction that utilizes an alkyne and a carbonyl compound as the starting materials, and the reaction typically performed in aqueous medium. Additionally, the reaction is sensitive to the presence of oxygen and moisture, which can deactivate the palladium catalyst and prevent the formation of the 1,3-diketone product..
References
- Glaser, C. (1869), Beiträge zur Kenntniss des Acetenylbenzols. [Contributions to the knowledge of acetenylbenzene.] Ber. Dtsch. Chem. Ges., 2: 422-424. https://doi.org/10.1002/cber.186900201183
- Glaser, C. (1870), Untersuchungen über einige Derivate der Zimmtsäure. [Studies on some derivatives of cinnamic acid.] Justus Liebigs Ann. Chem., 154: 137-171. https://doi.org/10.1002/jlac.18701540202
- Eglinton, G. and Galbraith, A. R., “Cyclic diynes” Chem. Ind., 1956, 737-738
- Eglinton, G., and Galbraith, A. R. (1959). Macrocyclic acetylenic compounds. Part I. Cyclotetradeca-1:3-diyne and related compounds. J. Chem. Soc., 889-896. doi: 10.1039/JR9590000889
- Oxidative Coupling of Acetylenes. II1
Allan S. Hay
The Journal of Organic Chemistry 1962 27 (9), 3320-3321
DOI: 10.1021/jo01056a511