What is Grignard reaction?
Grignard reaction is a chemical reaction that involves the addition of a Grignard reagent to a compound, resulting in the formation of a new carbon-carbon bond. This reaction was first reported by François Auguste Victor Grignard in 1900, and has since become a widely used synthetic method in the field of chemistry..
A Grignard reagent is a organomagnesium compound that is typically prepared by reacting an alkyl or aryl halide with magnesium metal. Grignard reagents are highly reactive and are able to function as nucleophiles, making them useful for synthesizing a wide range of compounds..
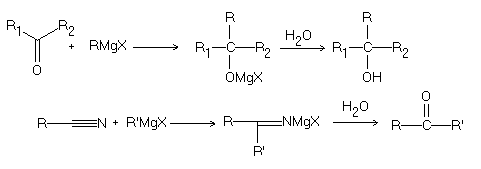
Grignard reaction typically involves the addition of a Grignard reagent to a compound, such as an aldehyde, a ketone, or an ester. The reaction is typically carried out in the presence of a solvent, such as ether or tetrahydrofuran, and a small amount of a catalytic amount of a halogen, such as iodine..
One of the key benefits of the Grignard reaction is that it allows for the synthesis of a wide range of products with a high degree of regioselectivity and stereoselectivity. This means that the reaction can be used to produce products with specific arrangements of substituents on the resulting product..
Grignard reaction has a wide range of applications in the synthesis of a variety of compounds, including pharmaceuticals, dyes, and materials. For example, it has been used to synthesize the anti-anxiety drug diazepam, as well as other compounds that have potential medicinal applications..
Summary
Grignard reaction is an important tool in the synthesis of a wide range of products and has a wide range of applications in the field of chemistry..
Example
The Grignard reaction is a carbon-carbon bond forming reaction between an organic halide and a metal organyl, such as magnesium, to form a new carbon-carbon bond. An example of the Grignard reaction is the reaction between ethyl bromide and magnesium to form ethylmagnesium bromide:
Ethyl bromide and magnesium are added to a flask containing an ether solvent such as diethyl ether..
The ethyl bromide reacts with the magnesium to form a magnesium halide salt (MgBr) and the organomagnesium compound, also known as the Grignard reagent, ethylmagnesium bromide..
The Grignard reagent can then be used to react with a carbonyl compound such as aldehyde or ketone to form an alcohol..
Once the reaction is complete, the magnesium halide salt is typically removed by aqueous acid..
Mechanism of reaction
The Grignard reaction is a carbon-carbon bond forming reaction between an organic halide and a metal organyl, such as magnesium, to form a new carbon-carbon bond. The mechanism of the Grignard reaction can be broken down into the following steps:
The organic halide, such as a bromoalkane, reacts with magnesium metal in the presence of an ether solvent to form a magnesium halide salt and an organomagnesium compound, also called Grignard reagent..
The Grignard reagent acts as a nucleophile, attacking the electrophilic carbon atom of an aldehyde or ketone. This forms a tetrahedral intermediate, which collapses to form a new carbon-carbon bond and regenerate the magnesium halide salt..
The product of the reaction is an alcohol, and the magnesium halide salt is typically removed by aqueous acid..
References
- V. Grignard, Compt. Rend. Acad. Sci. Paris 130, 1322 (1900)