What is Mislow-Evans rearrangement?
Mislow-Evans rearrangement is a [2,3]-sigmatropic rearrangement of allylic sulfoxides to allylic sulfenates which are captured by thiophiles to yield the allylic alcohols. Thus, effecting the 1,3-transposition of sulfoxide and alcohol functions.
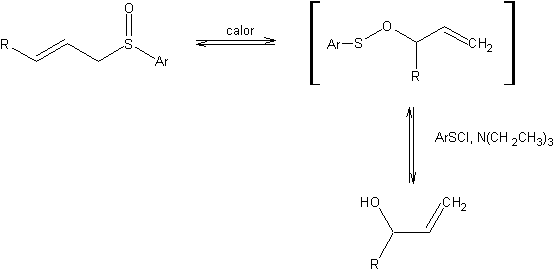
The reverse process is carried out by treating the alcohol with arylsulfenyl chloride, followed by thermal rearrangement of the sulfenate to generate the allylic sulfoxide.
References
- Thermal racemization of allylic sulfoxides and interconversion of allylic sulfoxides and sulfenates. Mechanism and stereochemistry
Paul Bickart, Frederick W. Carson, John Jacobus, Edward G. Miller, and Kurt Mislow
Journal of the American Chemical Society 1968 90 (18), 4869-4876
DOI: 10.1021/ja01020a021 - Reversible 1,3 transposition of sulfoxide and alcohol functions. Potential synthetic utilityD. A. Evans, G. C. Andrews, and C. L. SimsJournal of the American Chemical Society 1971 93 (19), 4956-4957
DOI: 10.1021/ja00748a075
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.