What is Pictet-Hubert reaction?
The Pictet-Hubert reaction is a chemical process, discovered in 1896, for creating phenanthridine by cyclizing and dehydrating N-acyl ortho-aminobiphenyls through heating with zinc chloride (ZnCl2) at temperatures ranging from 250-300 ºC. If phosphorus oxychloride POCl3 is used instead and the reaction is conducted in boiling nitrobenzene, it is referred to as the Morgan-Walls reaction or Morgan-Walls cyclization.
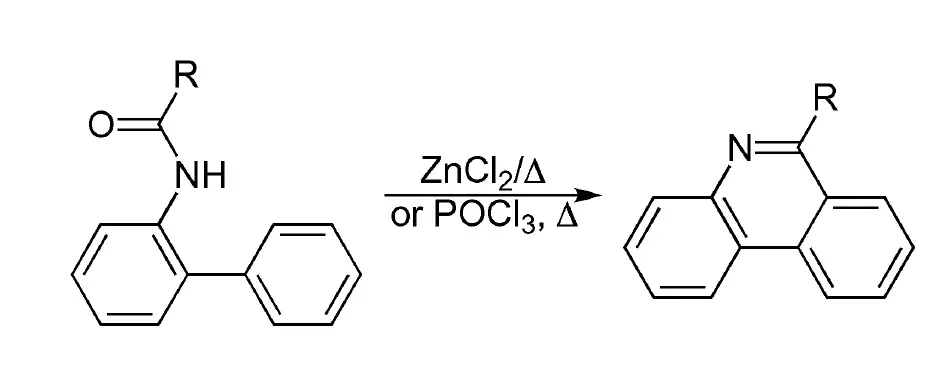
R = H, alkyl, aryl (see list of acronyms)
However, due to the low yield and lengthy reaction time associated with the original protocol, the Morgan-Walls condition using POCl3 as a dehydrating agent is more commonly used to prepare phenanthridine. This condition is also referred to as the Morgan-Walls cyclization. Although this condition was not successful in transforming ortho-formamidobiphenyl to the parent phenanthridine scaffold, it is still used.
To achieve optimal results, high-boiling solvents like nitrobenzene should be used. Additionally, the use of HBF4 as a catalyst has been shown to facilitate this cyclization.
References
- Pictet, A. and Hubert, A. (1896), Ueber eine neue Synthese der Phenanthridinbasen. [On a new synthesis of the phenanthridine bases] Ber. Dtsch. Chem. Ges., 29: 1182-1189. https://doi.org/10.1002/cber.18960290206
- G. T. Morgan, L. P. Walls, “CCCXXXV.—Researches in the phenanthridine series. Part I. A new synthesis of phenanthridine homologues and derivatives” J. Chem. Soc., 1931, 2447-2456
DOI: 10.1039/JR9310002447 - G. T. Morgan, L. P. Walls,”312. Researches in the phenanthridine series. Part II. Nitro- and amino-phenanthridines” J. Chem. Soc., 1932, 2225-2231
DOI: 10.1039/JR9320002225