What is Ramberg-Bäcklund rearrangement?
The Ramberg-Bäcklund rearrangement is a well-known transformation of α-halosulfones into olefins, which was first discovered by Ramberg and Bäcklund in 1940. This reaction is sometimes referred to as the Ramberg-Bäcklund reaction, Ramberg-Bäcklund elimination, Ramberg-Bäcklund olefination, Ramberg-Bäcklund synthesis, or Ramberg-Bäcklund olefin synthesis.
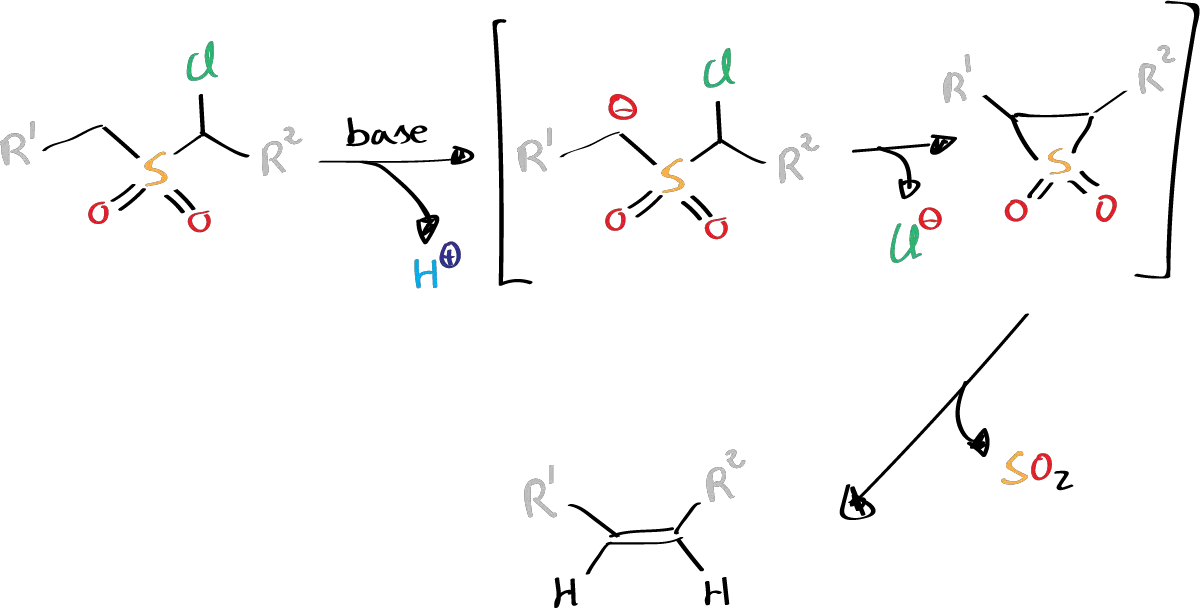
- R1 = alkyl, aryl
- R2 = H, alkyl, aryl (see list of acronyms)
It is a base-promoted reaction that occurs at mild conditions and involves the extrusion of SO2 via an episulfone intermediate. The reaction is applicable to almost all α-halosulfones that have at least one α-hydrogen. The mechanism of the reaction involves the deprotonation of α-hydrogen to generate a sulfonyl carbanion, which then undergoes an intramolecular nucleophilic displacement of α-halogen to form an episulfone intermediate. This intermediate is subsequently converted to the corresponding olefin.
The Ramberg-Bäcklund rearrangement usually gives the cis-elimination product and occurs at moderate temperatures, requiring only a dilute base. It is noteworthy that α-halosulfones are prone to 1,3-elimination to form episulfone when exposed to a base, regardless of the number of α-halogens. Furthermore, the base treatment of trichloromethyl sulfones leads to the formation of sulfonic acid.
The application of the Ramberg-Bäcklund rearrangement in organic synthesis is extensive, particularly for the synthesis of natural products and C-glycosides.
References
- Ramberg, L. and Bäcklund, B., “The reactions of some monohalogen derivatives of diethyl sulfone” Arkiv Kemi Mineral Geol., 1940, Band 13A, 1-50
- Ramberg, L. and Bäcklund, B., “The reactions of some monohalogen derivatives of diethyl sulfone” Chem. Abstr., 1940, 34, 4725
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.