Written by J.A Dobado | Last Updated on April 22, 2024
Preparation of derivatives
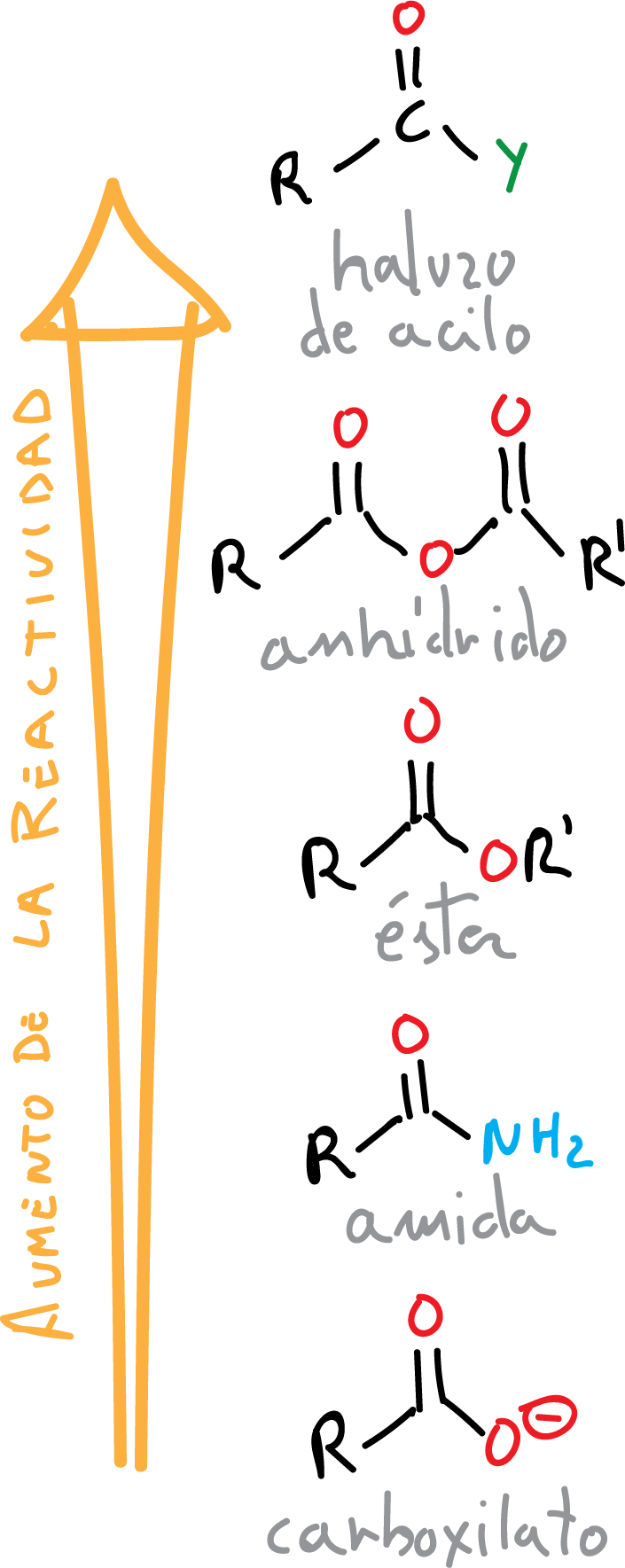
When the OH in a carboxylic acid is replaced by another function such as X (Cl, Br), OR, NH2, etc., compounds called “carboxylic acid derivatives” are obtained. All of them have in common the acyl group (R-C=O).
These derivatives are, in turn, converted to acids by hydrolysis, and also give rise to interconversion reactions between them. The reactions of formation of carboxylic acid derivatives proceed by a characteristic nucleophilic addition-elimination mechanism.
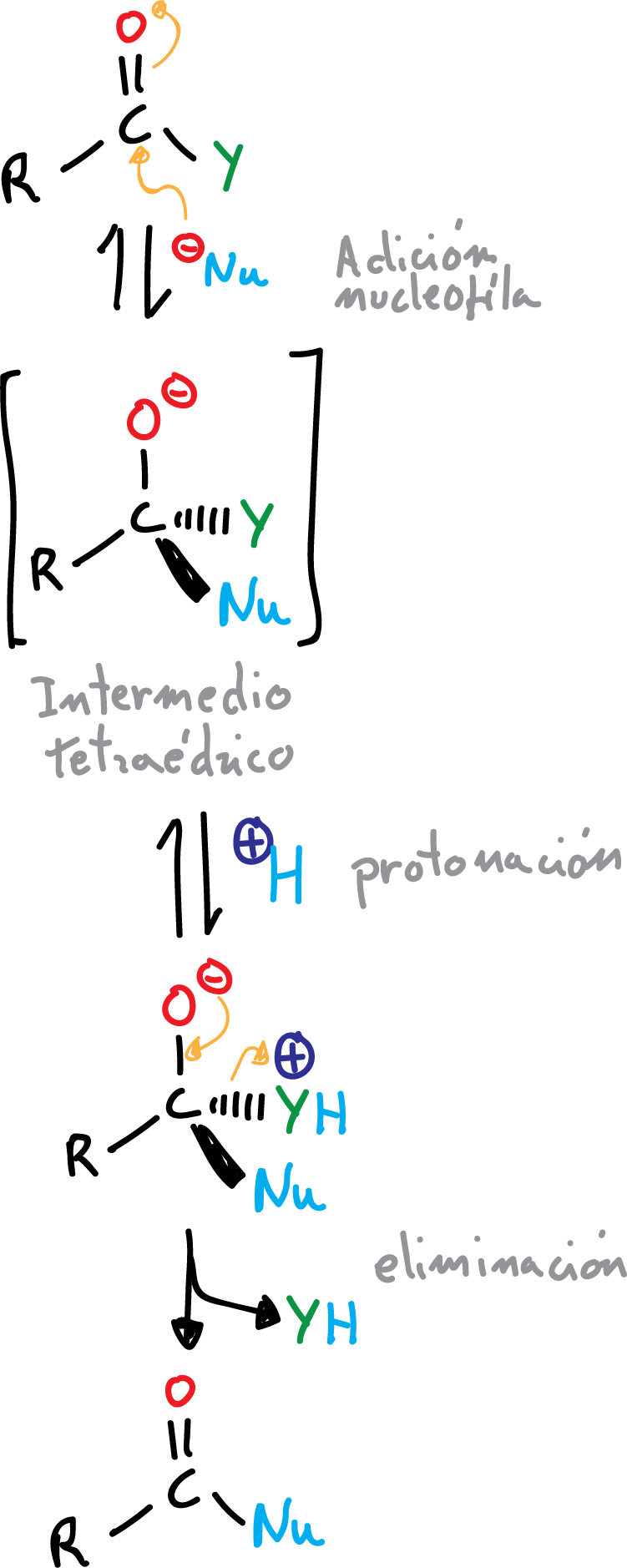
Unlike the reactions of aldehydes and ketones, where in the tetrahedral intermediate the -O⊖ is protonated, here one of the substituents is protonated (making them good leaving groups) and removed to regenerate the >C=O double bond.
The reactivity order of carboxylic acids in nucleophilic addition-elimination processes is explained according to the basicity of the leaving group, the most basic being the X⊖ anion and the least basic the H2N⊖ one, as well as by the resonance stabilization of certain groups:
Formation of acyl halide from carboxylic acid
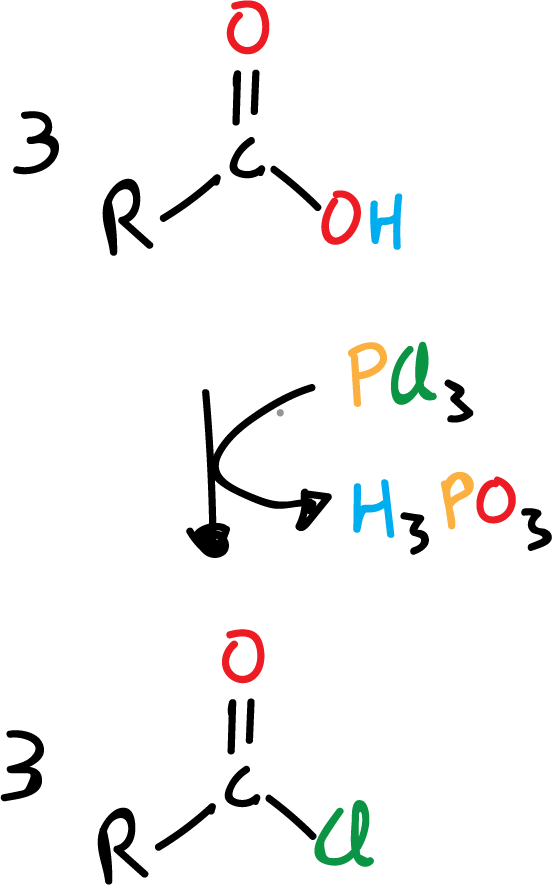
One of the most useful reactions of carboxylic acids is their conversion to acyl halides, specifically to acyl chlorides, since as we will see below these chlorides are very versatile and can, in turn, be converted to other derivatives easily. Frequently, one of the following reagents is used:
- thionyl chloride, SOCl2
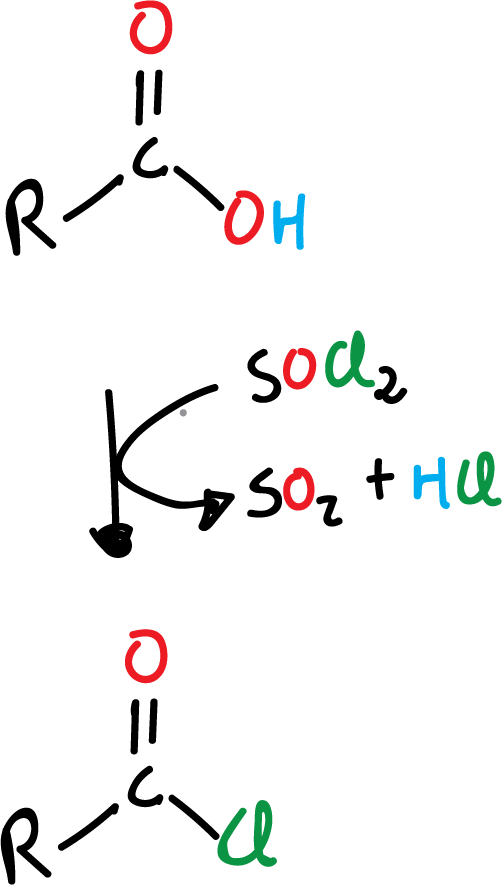
- phosphorus trichloride, PCl3
- phosphorus pentachloride, PCl5
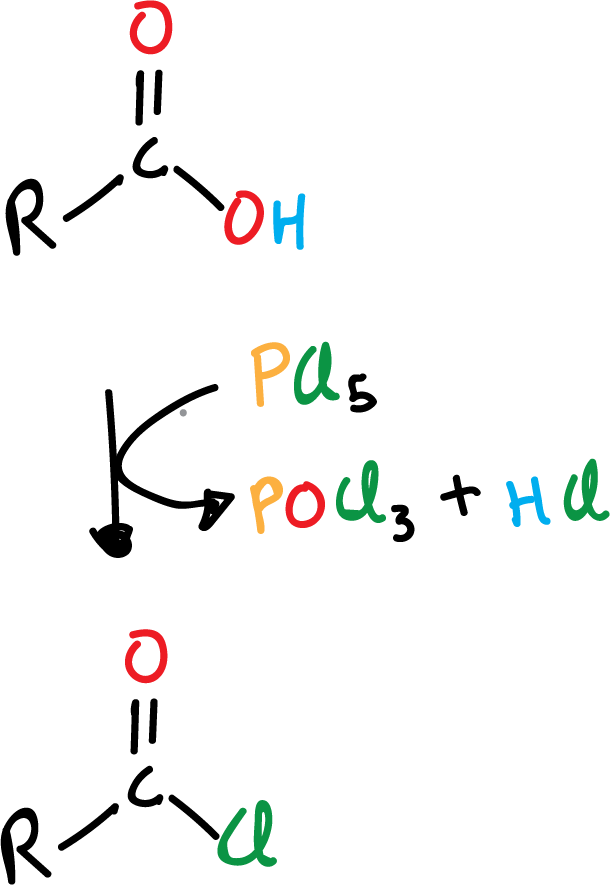
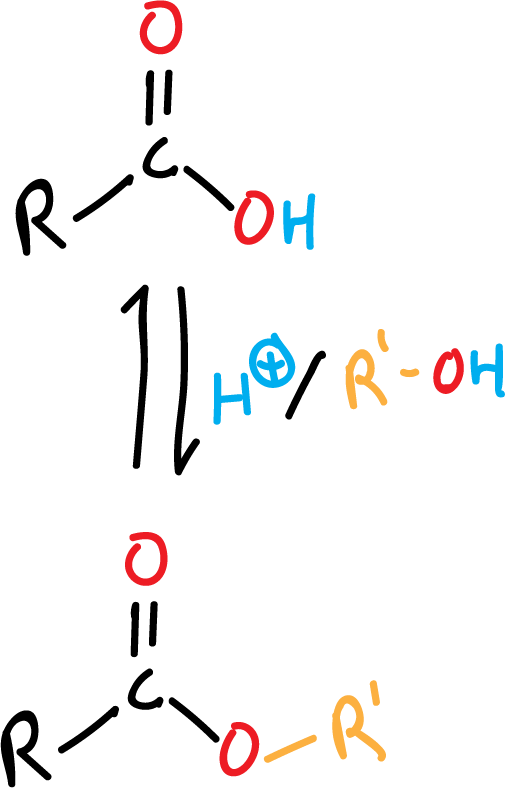
Ester formation from carboxylic acid (Fischer esterification)
Esters can be formed directly from their corresponding carboxylic acids by heating them with alcohol, under acid catalysis (usually concentrated H2SO4 or dry HCl).
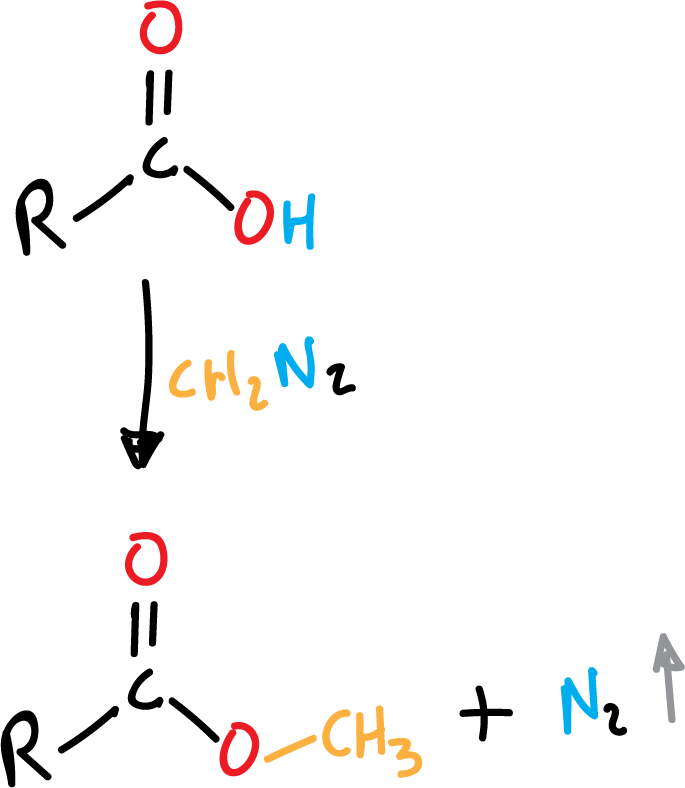
Ester formation from carboxylic acid
Methyl esters can be easily obtained from diazomethane (CH2N2) as shown in the scheme, as a reaction by-product gaseous nitrogen (N2) is obtained.
Formation of amide from carboxylic acid
Amides are obtained by replacing the -OH by the -NH2 group, from carboxylic acids. This is not done directly, but requires an additional reaction to form the corresponding acyl chloride. It is a two-step process, first the formation of the acyl chloride and then the formation of the amide.
With ammonia, the reaction is as shown in the following scheme:
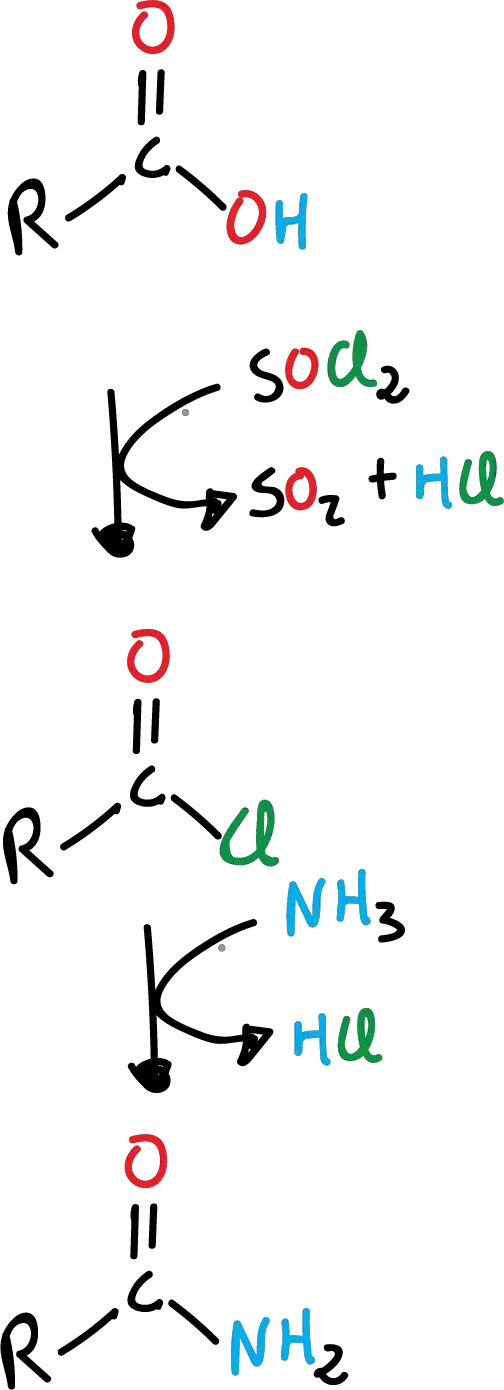
This reaction can also be carried out with primary amines (R’-NH2), as shown in the following scheme:
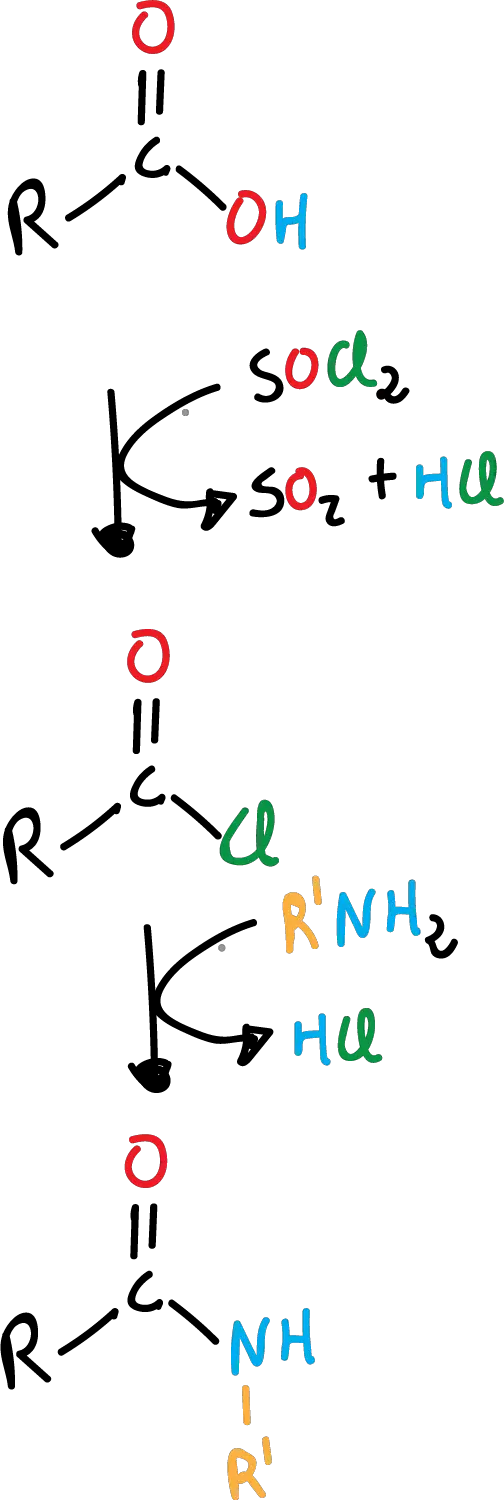
Formation of imide from dicarboxylic acid
Imides are the nitrogen analogs of acid anhydrides. One of the most characteristic imides is the phthalimide used in the synthesis of Gabriel.
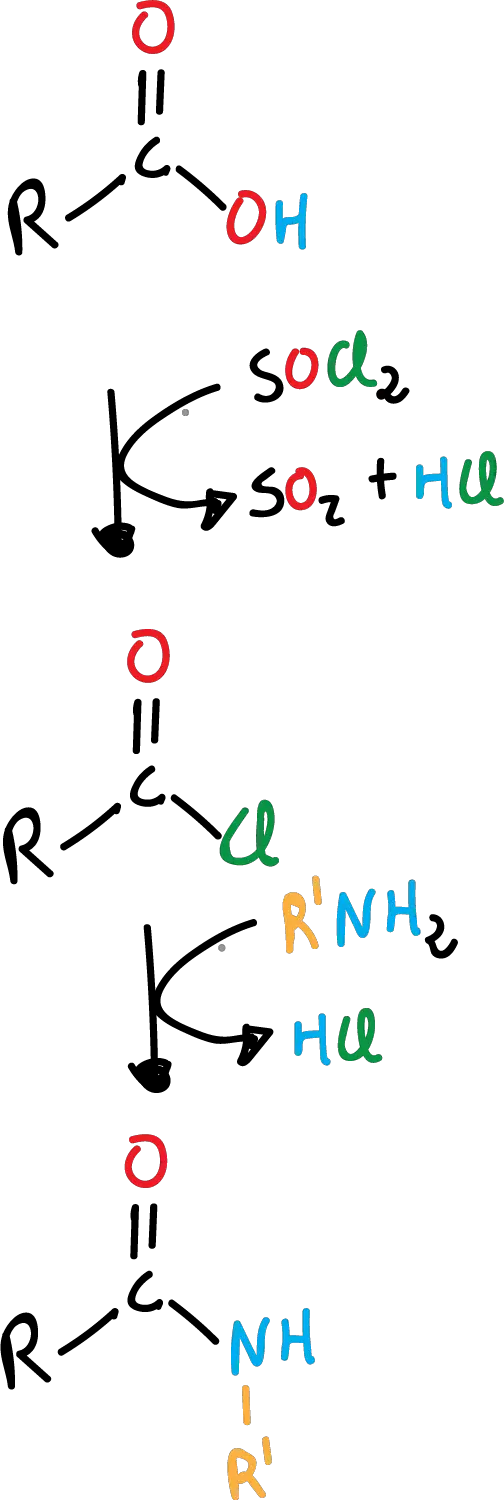
Reduction
One of the few reagents that reduces the carboxylic group to alcohol is LiAlH4. An alkoxide is generated which subsequently gives the alcohol by hydrolysis.
This reaction is especially useful in the transformation of long-chain carboxylic acids from fats. THF is usually used as solvent and the reaction is completed in slightly acidic aqueous medium.
fig9
Halogenation at α-position
Aliphatic carboxylic acids react under mild conditions with halogens at carbon-α in the presence of small amounts of phosphorus (Hell-Volhard-Zelinsky reaction).
fig10
Acyl chlorides
Formation of anhydride from acyl chloride
Acyl chlorides are the most reactive carboxylic acid derivatives. Acid anhydrides are obtained by the reaction of acyl chloride with carboxylic acid in the presence of a non-nucleophilic base (e.g. pyridine), which is used to neutralize excess HCl.
fig11
Ester formation from acyl chloride
A very useful way to obtain an ester is from the reaction of an acyl chloride with an alcohol. This is done under basic conditions, to neutralize the excess HCl.
fig12
Reduction to acyl chloride aldehydes (Rosenmund reduction)
Catalytic hydrogenation of acyl chlorides yields aldehydes. The catalyst must be poisoned with BaSO4and quinoline.
fig13
Reduction to aldehyde from acyl chloride
To achieve partial reduction to aldehyde we need to use hydride transfer reagents featuring bulky alkyl groups. Since LiAlH4 and NaBH4 would also react with the aldehyde obtained, modified reagents such as LiAl(t-BuO)3H (with a hydride that reacts only with the acyl chloride and not with the aldehyde formed) are used.
fig14
Reduction to primary alcohol from acyl chloride
With LiAlH4, the acyl chlorides are fully reduced to primary alcohol.
fig15
Formation of tertiary alcohol from acyl chloride
With carbon nucleophiles such as Grignard reagents (R’MgX), acyl chlorides are directly reduced to tertiary alcohol (the corresponding ketone is not obtained).
fig16
Formation of amide from acyl chloride
Ammonia as well as primary and secondary amines react with acyl chlorides to give amides. Basic conditions are used, as in ester formation, to neutralize the HCl formed.
fig17
Hydrolysis to carboxylic acid from acyl chloride
Acyl chlorides hydrolyze to their corresponding carboxylic acids by an addition-elimination mechanism. They are so reactive that just the addition of cold water hydrolyzes them. Aliphatic acyl chlorides react much more rapidly (sometimes violently) than their aromatic counterparts.
fig18
Ketone formation from acyl chloride
The transformation of an acyl chloride to ketone could be performed with organometallic reagents (RLi) and Grignard reagents (RMgX); however, because these are not very selective they react with the ketone formed giving alcohol.
Therefore, the best method of obtaining ketones from acyl chlorides is by organocuprate compounds. These are more selective than RLi or RMgX and do not react with the ketone obtained.
fig19
Ketone formation from acyl chloride (Friedel-Crafts acylation)
fig20
Acid anhydrides
Conversion to carboxylic acid
Acid anhydrides give rise to substitution reactions analogous to those studied for acyl chlorides, but less vigorously. In this case the leaving group is a carboxylate ion instead of the chloride.
Basic conditions are used to remove the carboxylic acid that is obtained as a by-product in some of these reactions. The anhydrides are hydrolyzed to give the corresponding carboxylic acids. In addition, the cyclic anhydrides give rise to ring breaking producing dicarboxylic acids.
fig21
Ester formation
By gently heating an anhydride in the presence of an alcohol, an ester is obtained. In addition, carboxylic acids are generated as by-products, which can be removed in a basic medium. In the case of cyclic anhydrides the following acid derivative is obtained:
fig22
Primary alcohol formation
The acid anhydrides react with LiAlH4, being totally reduced to primary alcohol, by the addition of two moles of hydride.
fig23
Amide formation
Anhydrides react with ammonia to give an amide -CONH2 and the ammonium salt of the corresponding acid. Similarly, they also react with primary and secondary amines to give N-substituted and N,N-disubstituted amides, respectively.
fig24
fig25
Formation of ketones (Friedel-Crafts acylation)
(See aromatic substitution reactions in benzene and derivatives.)
Esters
Acid or basic hydrolysis
Hydrolysis with water is so slow that, alternatively, reflux heating with acid catalysis (HCl or H2SO4diluents) is used. The reactions are equilibrium reactions.
A better alternative to this reaction is obtained with a dilute base as catalyst (NaOH). The advantage is that with basic catalysis the reaction is not equilibrium and the products are easier to separate.
fig26
Amide formation
Reaction with ammonia converts the esters to amides. It can be generalized for any 1º primary or 2º secondary amine, giving rise to N-substituted and N,N-disubstituted amides, respectively.
fig27
Transesterification
Esters react with alcohols to give other esters, where the alkyl group comes from the corresponding alcohol. This ester interconversion reaction is called transesterification.
fig29
Saponification
It consists of the basic hydrolysis of an ester to give the corresponding alcohol and the carboxylic acid salt. It is popularly used in the manufacture of soap from fat or oil by carrying out the reaction with an alkaline base (NaOH or KOH).
fig29
Reduction to primary alcohol
Esters can be reduced into primary alcohols using hydride transfer reagents (LiAlH4), or by hydrogenation with copper oxide as a catalyst.
fig30
Tertiary alcohol processing
With Grignard reagents (R’MgX), carboxylic esters are transformed into tertiary alcohols.
fig31
Claisen condensation
(See Claisen condensation in enolate reactions.)
Amides
Anions formation
Amides practically do not occur in acyl transfer reactions, since they form resonant structures where the positive charge is placed on the nitrogen instead of the carbonyl carbon.
fig33
Acid formation
Amides can revert to carboxylic acids by acid hydrolysis. The C-N bond is broken, regenerating the corresponding carboxylic acid. The reaction is carried out by heating the amide using acid or basic catalysis.
With acid catalysis the carboxylic acid is obtained in the form of ammonium salt, whereas with basic catalysis carboxylate ions are formed.
fig34
amine formation (Hofmann degradation)
Amides can be transformed into amines by treatment with bromine in a basic medium. The resulting amine decarboxylates with one less carbon.
fig40
Amine formation
Unlike the previous case with LiAlH4, the amides give amines conserving the same carbon chain length.
fig40
Nitriles
Carboxylic acid formation
Nitriles, like the derivatives in this section, hydrolyze to carboxylic acids.
fig40
Reduction
The use of hydride transfer reagents such as LiAlH4, reduces nitriles to their corresponding amines.
fig40
Transformation into aldehydes
This reaction is carried out with hydride transfer reagents such as DIBAH (diisobutyl aluminum hydride).
fig40
Transformation into ketones
The nitriles are converted to the corresponding ketones with Grignard reagents (R’MgX), via an anionic intermediate undergoing hydrolysis.
fig40