Written by J.A Dobado | Last Updated on April 22, 2024
The most significant reactions in organic chemistry of the functional groups alcohols, ethers and oxiranes are described below.
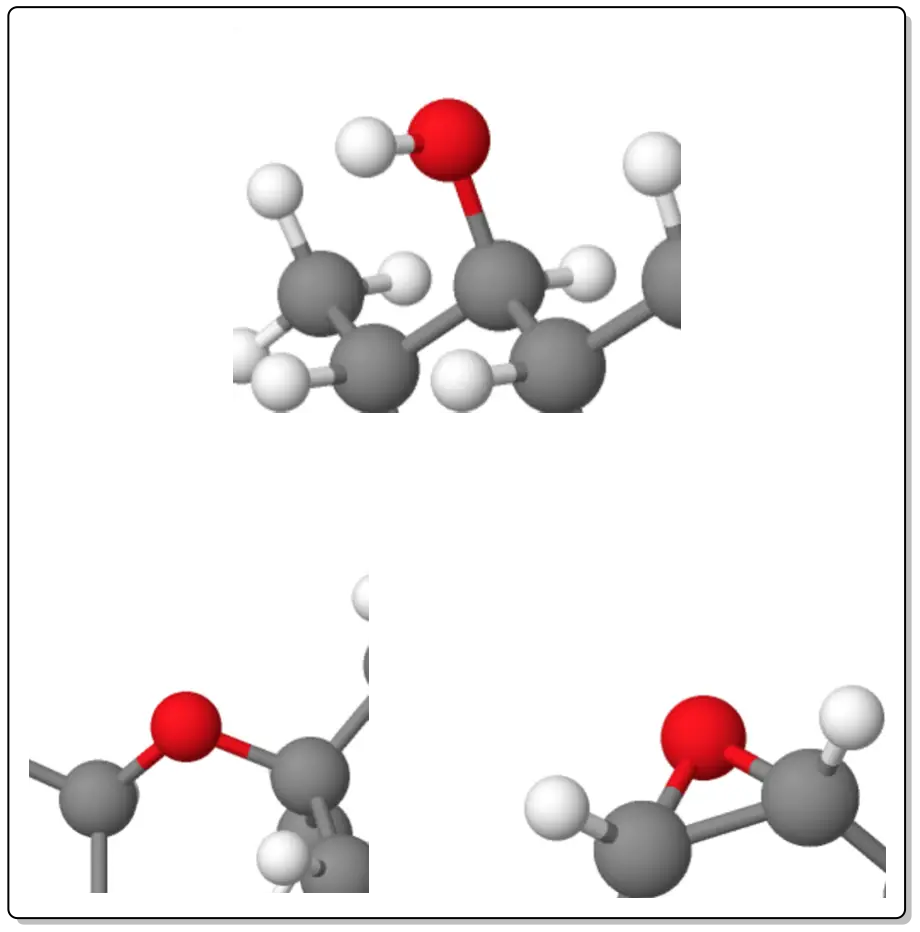
Dehydration of alcohols
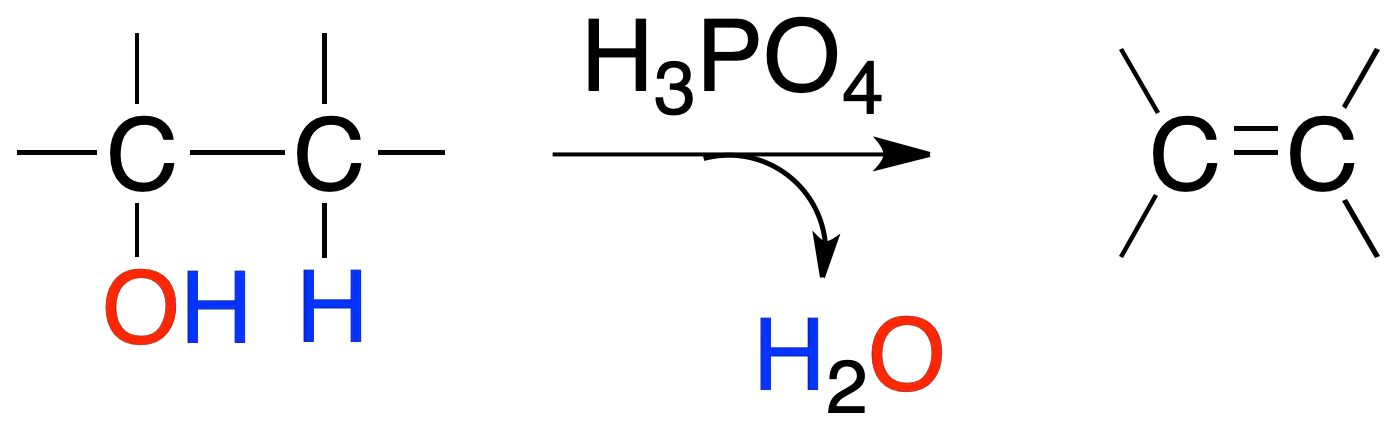
Alcohols in the presence of mineral acids suh as H2SO4 o H3PO4 they give alkenes by loss of a water molecule. The order of reactivity of alcohol is:
tertiary (3º) > secondary (2º) > primary (1º)
The process is regioselective on secondary and tertiary alcohols.
The reactions obey Zaitsev’s rule which states “the more highly substituted alkene is the more likely product formed in an elimination reaction“.
The explanation lies in the fact that this is the most thermodynamically stable alkene.
Tertiary and secondary alcohols react by a E2 mechanism, whereas in primary alcohols it is of the E1 type, due to the instability of the hypothetical primary carbocation that would occur.
Sometimes alkenes are produced from the transposition of carbocations (as reaction intermediates). HX-type acids are not used in these reactions due to the nucleophilic character of the anion, thus avoiding substitution reactions.
Conversion of alcohols to ethers
Conversion of alcohols to ethers in acidic media
Primary alcohols are transformed into ethers by acid catalysis (heating).
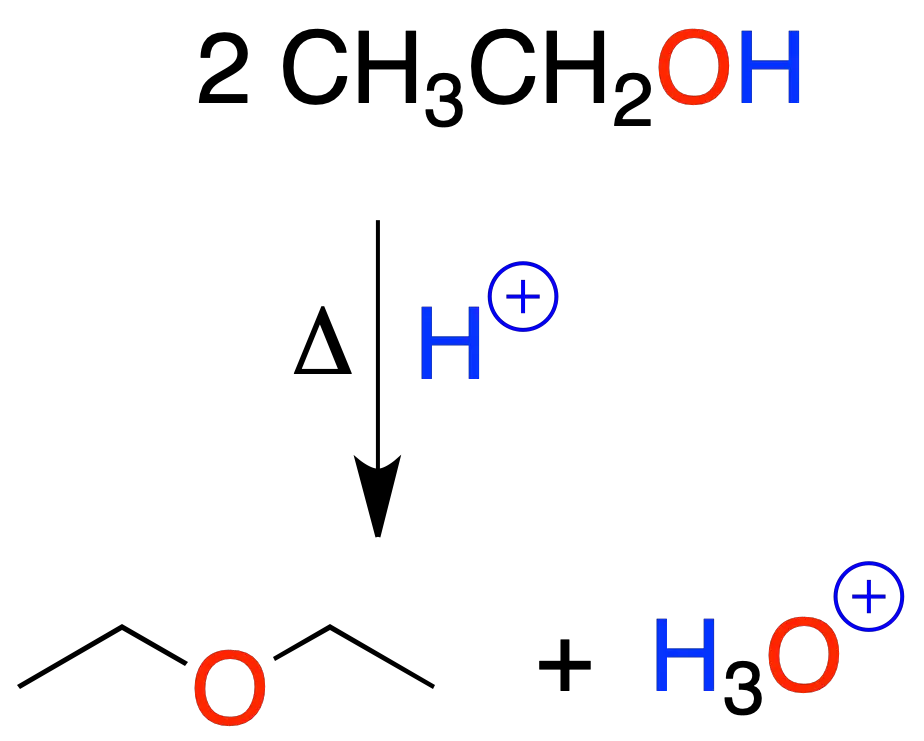
Generally, sulfuric acid is used. The reaction starts by protonation of the hydroxyl of the alcohol.
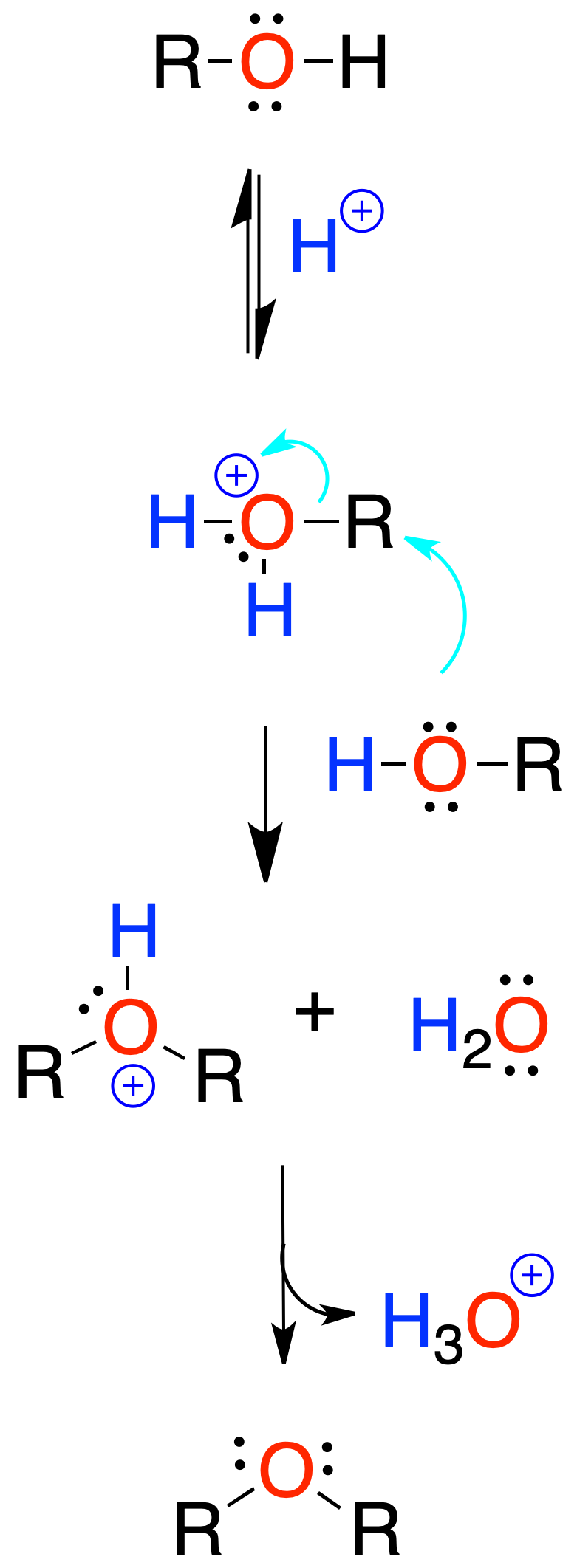
It is limited to the preparation of symmetric ethers from primary alcohols.
Under these conditions secondary and tertiary alcohols preferentially give alkenes.
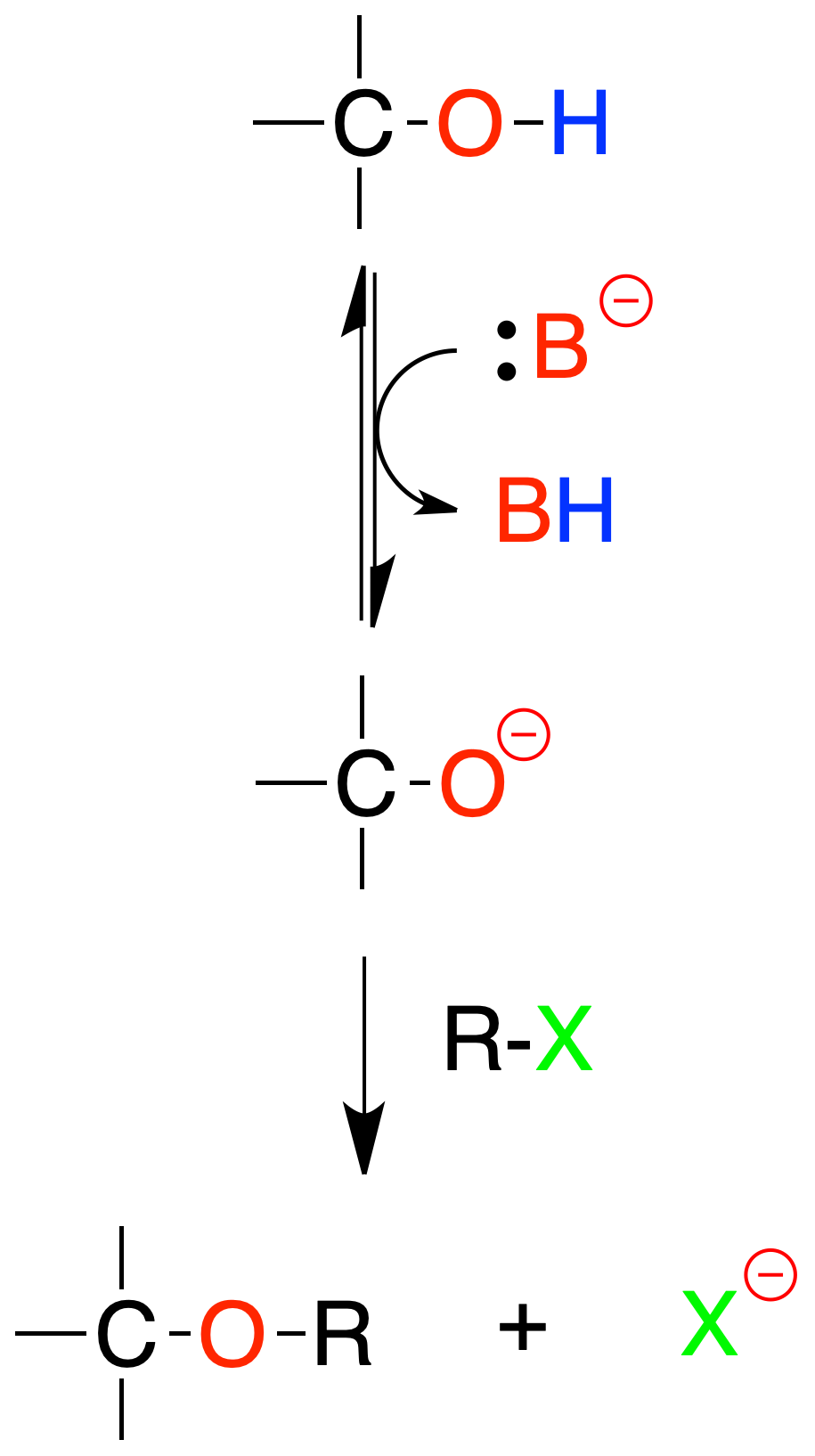
Conversion of alcohols to ethers by means of alkoxides (Williamson’s synthesis)
Treatment of an alcohol with a suitable base generates an alkoxide which by nucleophilic displacement on an alkyl halide produces an ether. The base used with the alcohol is Nao.
The alcohol can be primary, secondary or even tertiary. However, the alkyl halide must be methyl or primary since the mechanism of this reaction is SN2 type
In secondary and tertiary halides the elimination reaction predominates to give alkenes, so the procedure is not applicable.
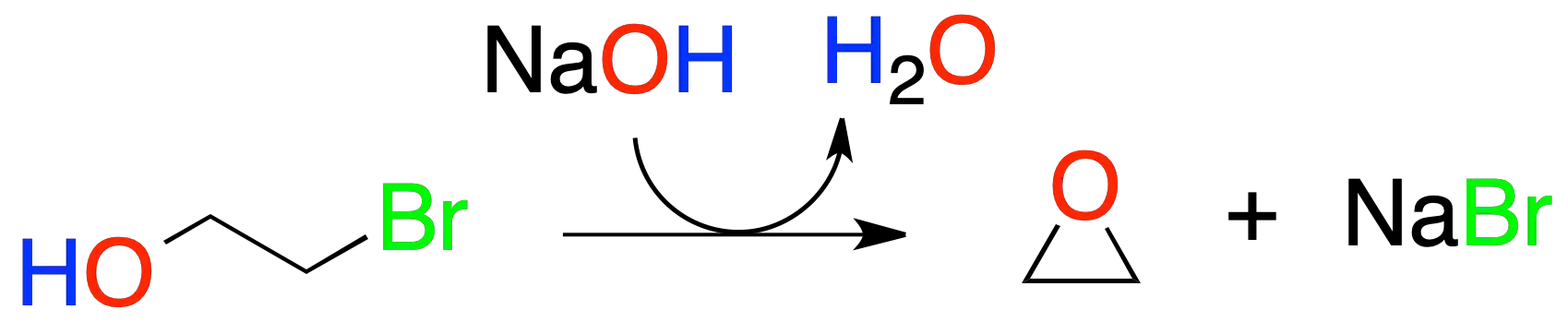
If this reaction is performed intramolecularly, it gives rise to cyclic ethers, e.g., a halohydrin forms the corresponding epoxide.
Conversion of alcohols to silyl ethers
The alcohols react with trialkylsilyl chlorides to give silyl esters, in the presence of a tertiary amine such as triethylamine, pyridine or imidazole which acts as a base and neutralizes the HCl released.
These compounds are stable under a wide variety of conditions, such as in the presence of oxidants, reductants, or acids and bases in aprotic media.
They are easily cleaved with fluoride ion in the form of tetralkyl ammonium salt. Silyl derivatives (TMSO-, TESO-, TIPSO- or TBDMSO-), are used as protective group of hydroxyls in many syntheses.
Conversion of alcohols to esters
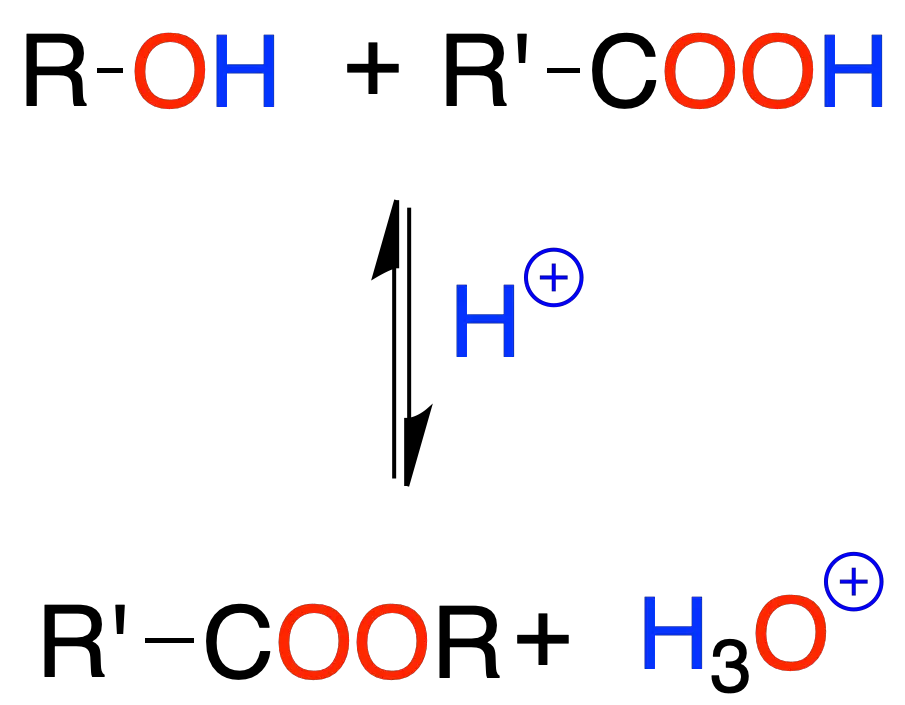
Carboxylic acid esters (Fischer’s esterification)
Alcohols with carboxylic acids react to give esters in the presence of a mineral acid.
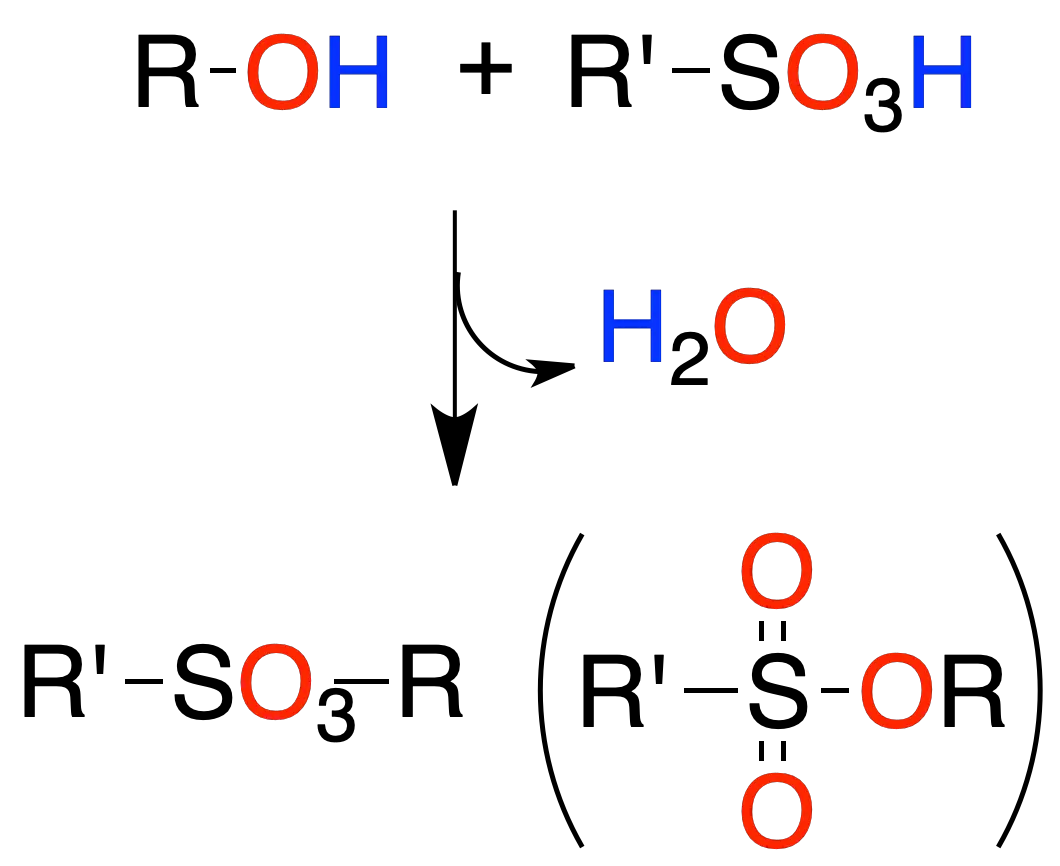
Esters of sulfonic acids
The reaction of an alcohol with sulfonic acids leads to the formation of a sulfonic ester.
Conversion of alcohols to haloalkanes
Reactions of alcohols with HX
Alcohols react with HX to yield alkyl halides.
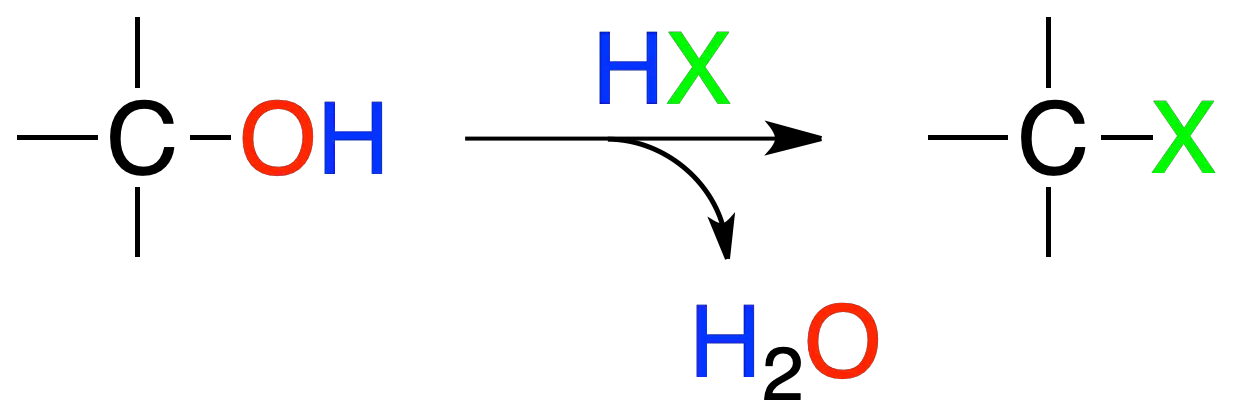
The order of reactivity for hydrogen halide is HI > HBr > HCl > HF, while for alcohols it is:
tertiary (3º) > secondary (2º) > primary (1º) > methyl
The reaction generally proceeds by an SN1-type mechanism, so that in some cases transpositions in the intermediate carbocations may occur.
For primary alcohols or methanol the reaction mechanism is of the SN2 type.
Primary and secondary alcohols do not react with HCl at the rate necessary to give good yields. Secondary alcohols (2º) that do not react with HCl, do react when a solution of ZnCl2 in HCl concentrate is used (Lucas reagent).
With HBr, in secondary alcohols acceptable or good yields are obtained if the temperature is raised. The best result is obtained with tertiary alcohols.
Reactions of alcohols with thionyl chloride (SOCl2)
Alcohols react with thionyl chloride (SOCl2) to give alkyl chlorides and as by-products, sulfur dioxide (SO2) and hydrogen chloride HCl.
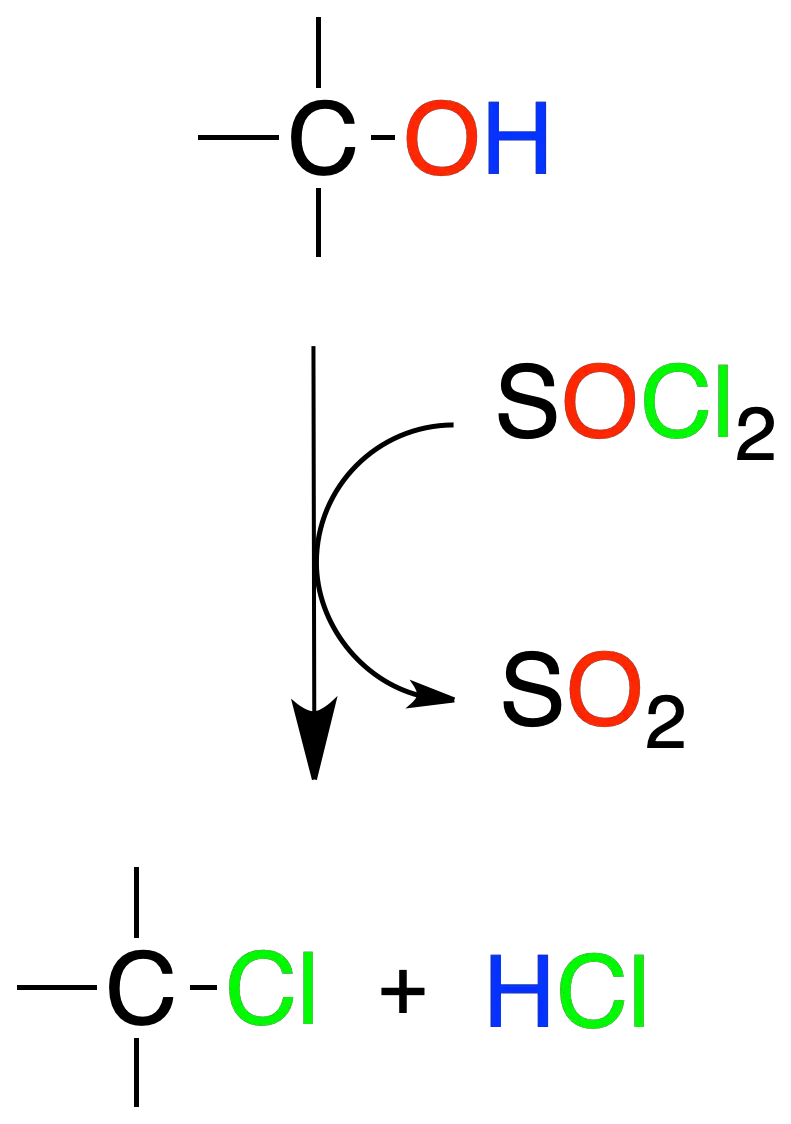
Esta reacción se aplica, fundamentalmente, en alcoholes primarios y secundarios, y transcurre mediante un intermedio clorosulfito (R-O-SO–Cl).
Depending on whether or not a base such as pyridine is used, the reaction proceeds with inversion or with retention of the configuration.
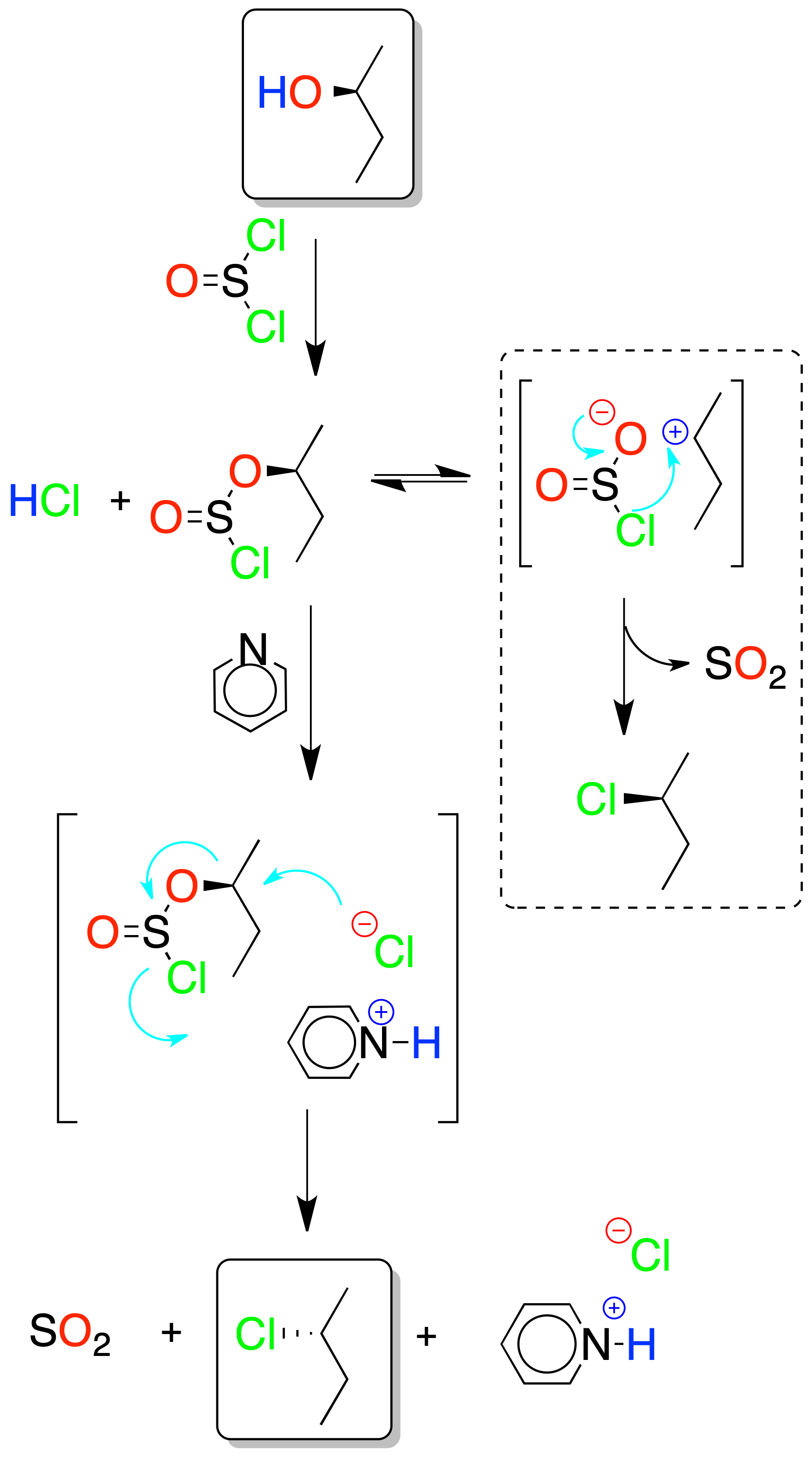
With base, a sufficient concentration of Cl⊕ (in the form of pyridinium chloride) is available for chlorosulfite displacement to occur by an SN2 mechanism. If there is no base, the reaction proceeds with retention of the configuration by an intramolecular process (syn).
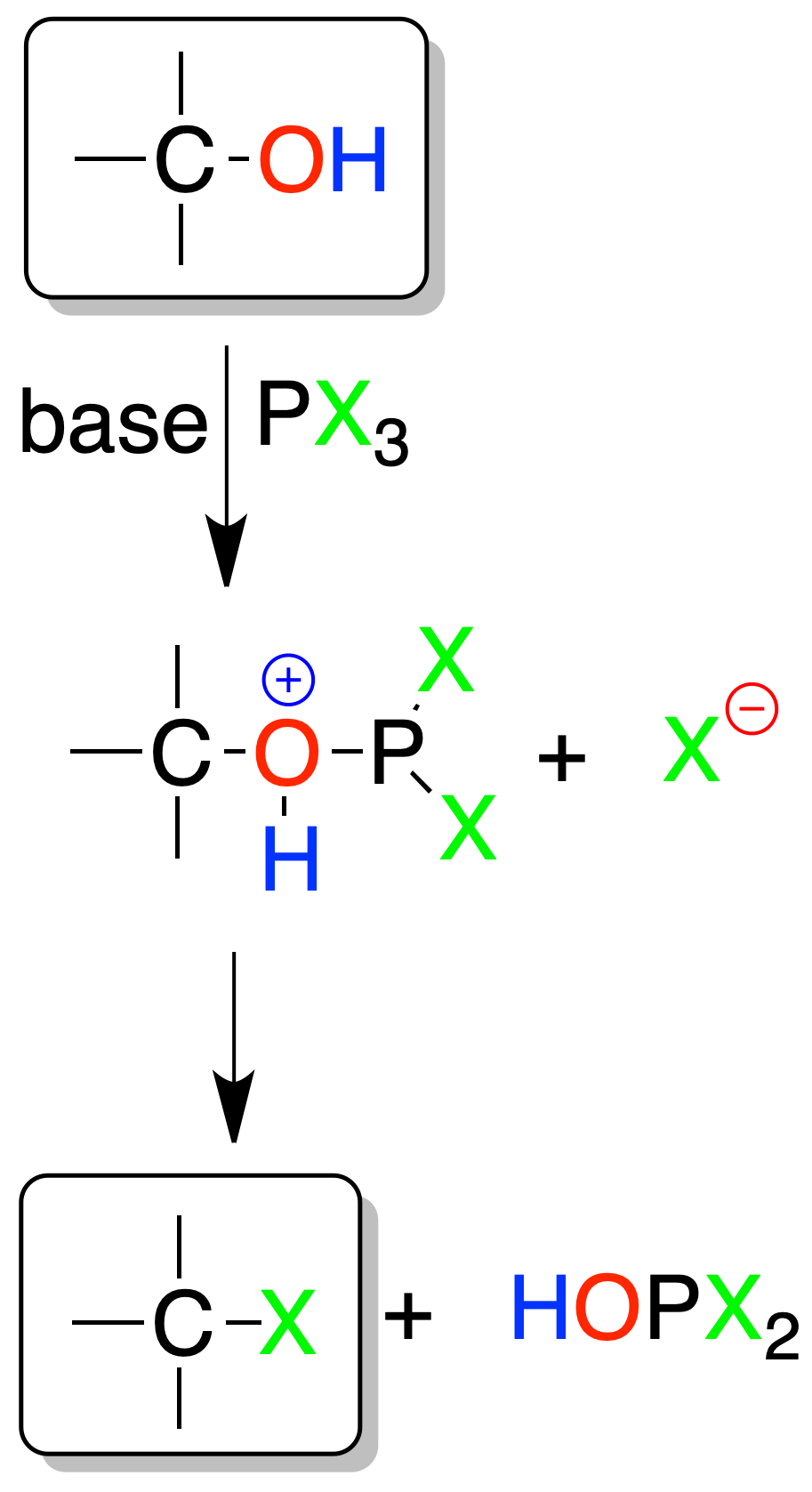
Reaction of alcohols with phosphorus trichloride or tribromide
Los alcoholes se transforman en haluros de alquilo con tricloruro o tribromuro de fósforo. The reaction occurs preferentially in primary alcohols and the mechanism is SN2. In secondary alcohols SN1 competes and skeletal transposition products are obtained. One mole of PX3 reacts with three moles of alcohol.
Reaction of alcohols with CX (Appel reaction).
The alcohols react with tetrahalomethanes (X = Cl or Br) and triphenylphosphine to give, in good yields, the corresponding alkyl halide with configuration inversion. The reaction conditions are mild and compatible with a multitude of functions.
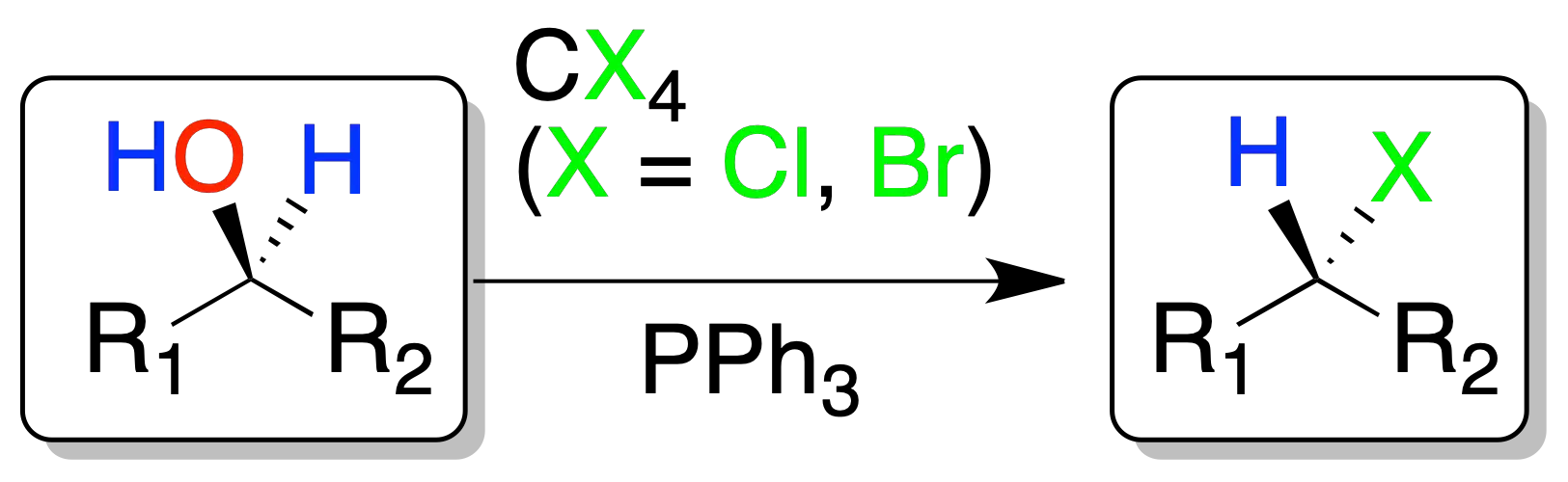
| Substrate | Stereochemistry / mechanism | Reagent / reaction conditions |
| Alcohol 2º and mainly 3º | Racemization / SN1 | XH |
| Alcohol 1º | Inversion of configuration / SN2 | PX3 / in the presence of base |
| Alcohol 1º and 2º | SOCl2 / in the presence of base | |
| CX4 / PPh3 | ||
| Retention of configuration / SNi | SOCl2 / in the absence of base |
Deoxygenation of alcohols (reduction)
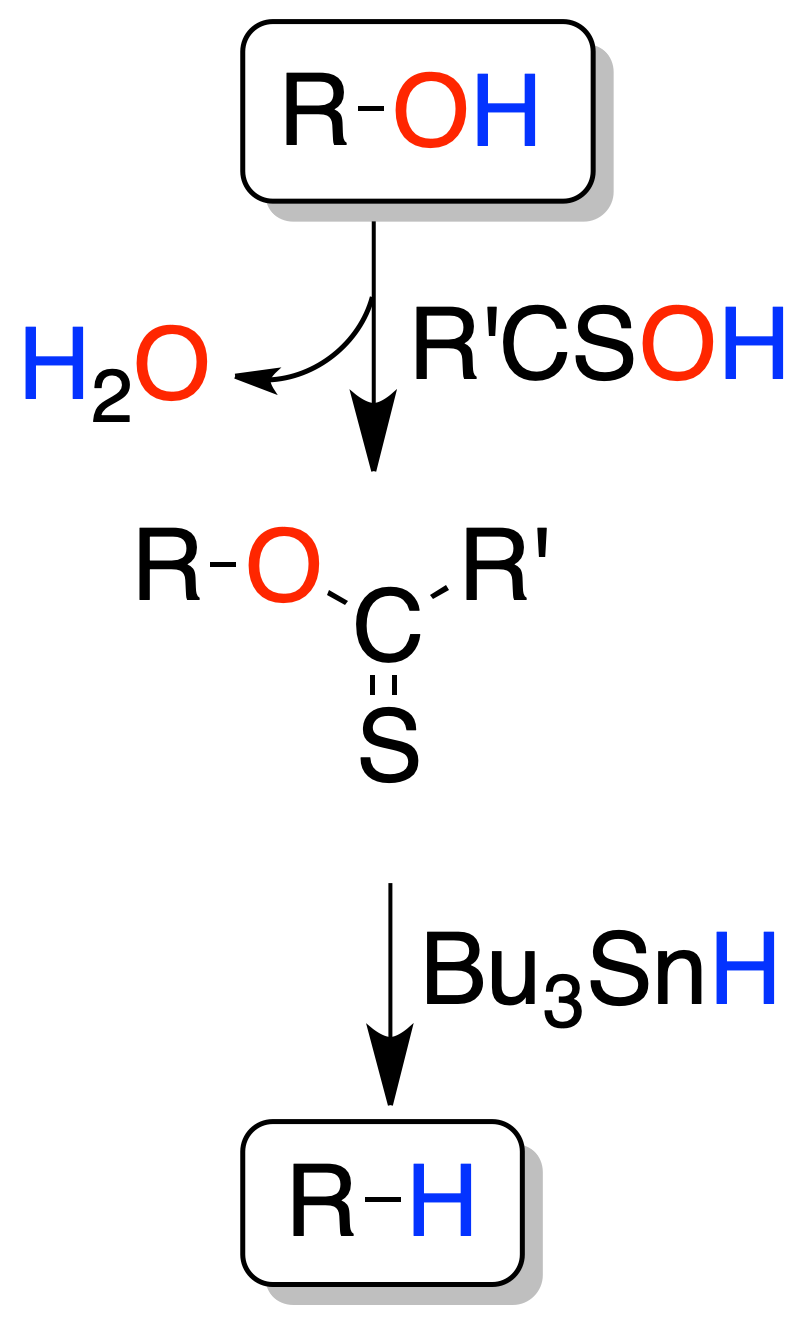
By means of thiocarbonyl derivatives (Barton-McCombie reaction)
Normally, alcohols cannot be directly transformed into the corresponding saturated compounds. Generally, it is necessary to carry out the transformation in two steps.
In the first instance an alcohol derivative is obtained, and on this derivative the transformation into the saturated compound takes place.
In this deoxygenation method an alcohol is transformed into thiocarbonyl derivative, which subsequently reacts with tri-butyltin hydride (Bu3SnH) by a radical process.
Sulfonate-mediated reactions
The alcohol is first transformed into a sulfonate (tosylate or mesylate) which, by treatment with LiAlH4 is transformed into the saturated compound.
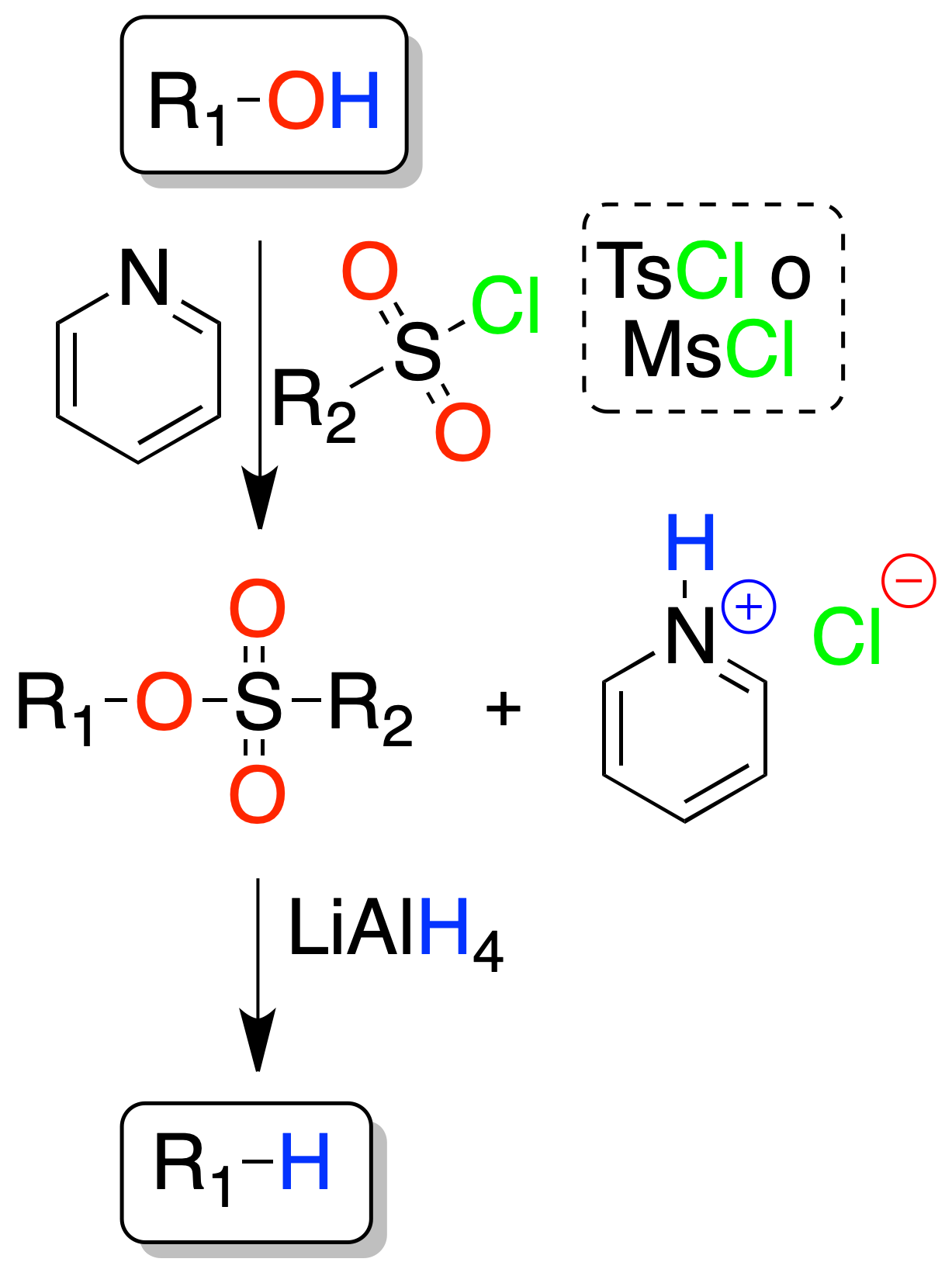
The reaction proceeds in good yields from primary (1º) and secondary (2º) alcohols.
Oxidation of alcohols
Alcohols can be oxidized by the reactions shown in the general scheme. For oxidation to occur, at least one hydrogen atom must be present on the carbon to be oxidized (1º primary or 2º secondary alcohol), so that a C=O double bond can be formed. Therefore, tertiary alcohols do not react.
| 2º Alcohol → ketones and 1º Alcohol → aldehydes | |
| Oxidant [O] | Solvent |
| PCC | CH2Cl2 |
Collins reagent (CrO3 2 pyridine) | |
Swern reagent ((ClCO)2 / DMSO) | CH2Cl2 or ethers |
| 2º Alcohol → ketones and 1º Alcohol → carboxilic acids | |
Jones reagent (H2CrO4) | acetone / H2SO4 |
| KMnO4 in a basic medium | H2O with a solvent mixture |
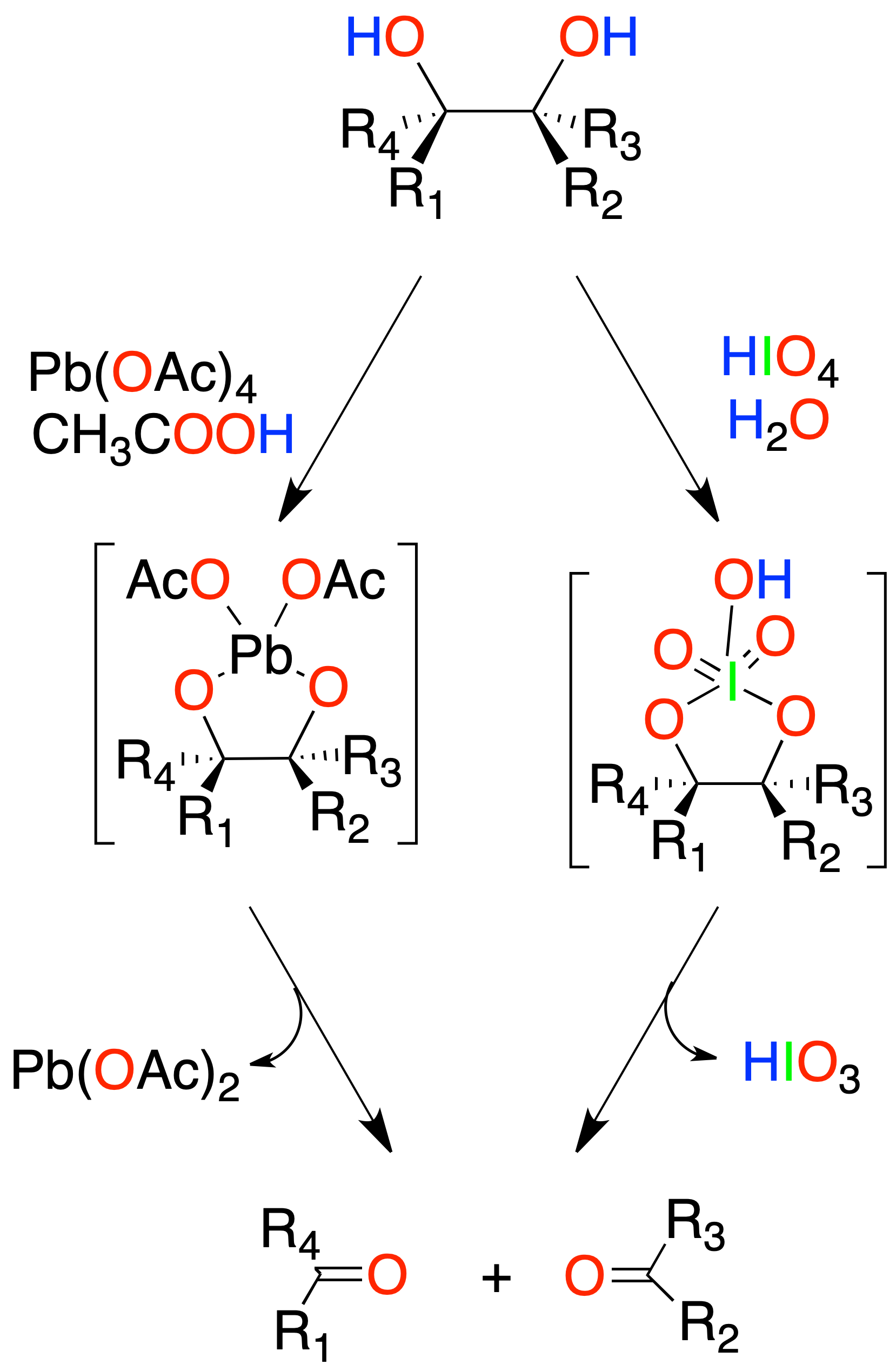
Oxidation of 1,2-diols
The neighboring diols can be subjected to an oxidative cleavage process with periodic acid (HIO4) or with lead tetra-acetate to give two carbonyl compounds.
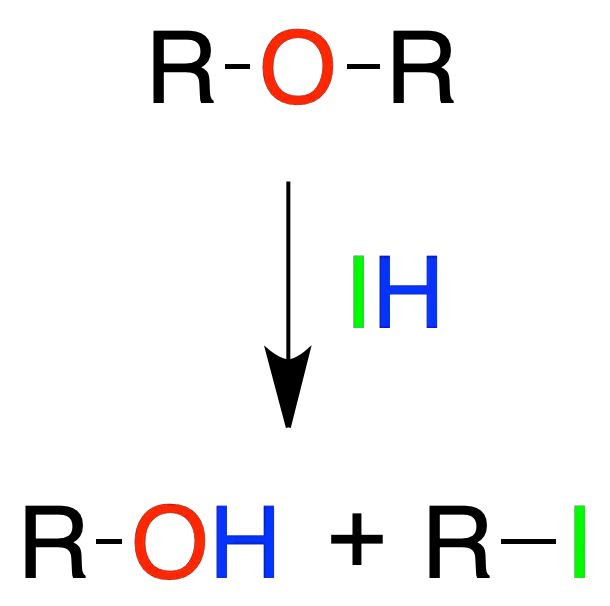
Ethers breaking
Ether cleavage with acids of the HX type occurs at room temperature when X = I. For Br it is necessary to increase the temperature to about 100 °C, whereas even higher temperatures are required for Cl.
Ring-opening of oxiranes
Acid catalyzed
When an oxirane (epoxide, oxacyclopropane) is treated with a mineral acid, protonation of the oxygen atom occurs, which facilitates ring opening by attack of a nucleophile.
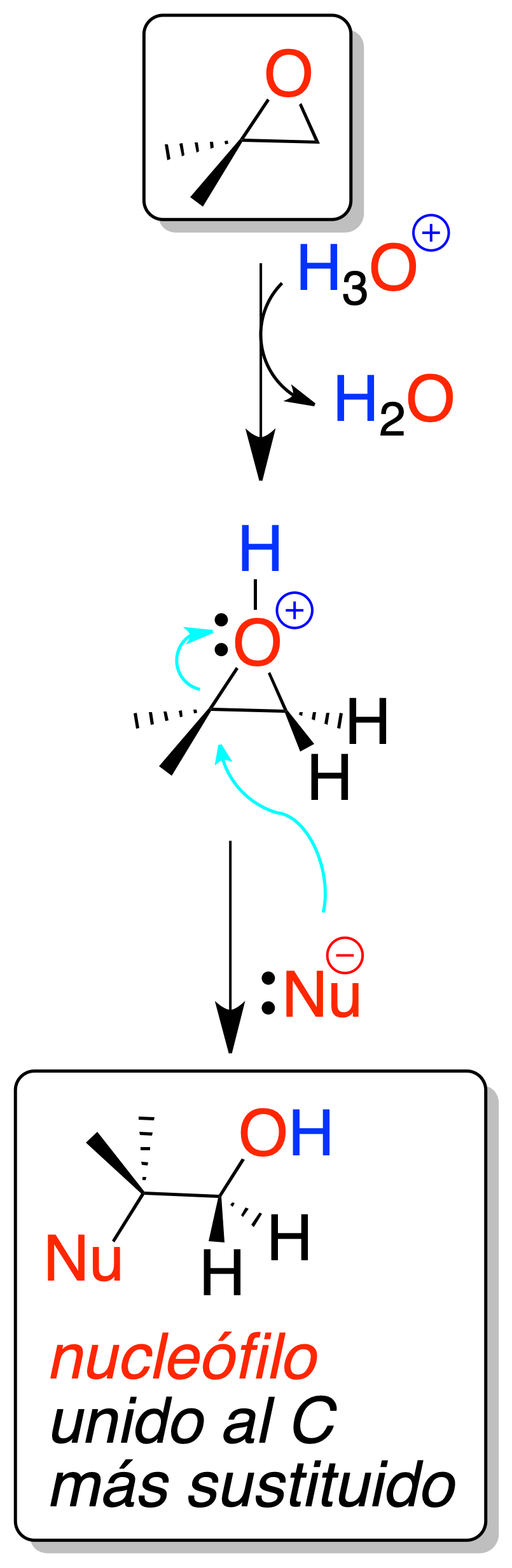
In all cases, the attack of the nucleophile is regioselective, since it occurs on the most substituted carbon. The reaction mechanism is of the SN1-process type.
The main reactions are the three indicated in the scheme:
- Ring opening in aqueous medium to obtain 1,2-glycols.
- Ring opening in the presence of alcohols to obtain hydroxyethers.
- Ring opening with hydrogen halides to yield halohydrins.
Nucleophilic
Nucleophiles can open oxiranes by attack on the least substituted carbon atom (regioselective reaction).
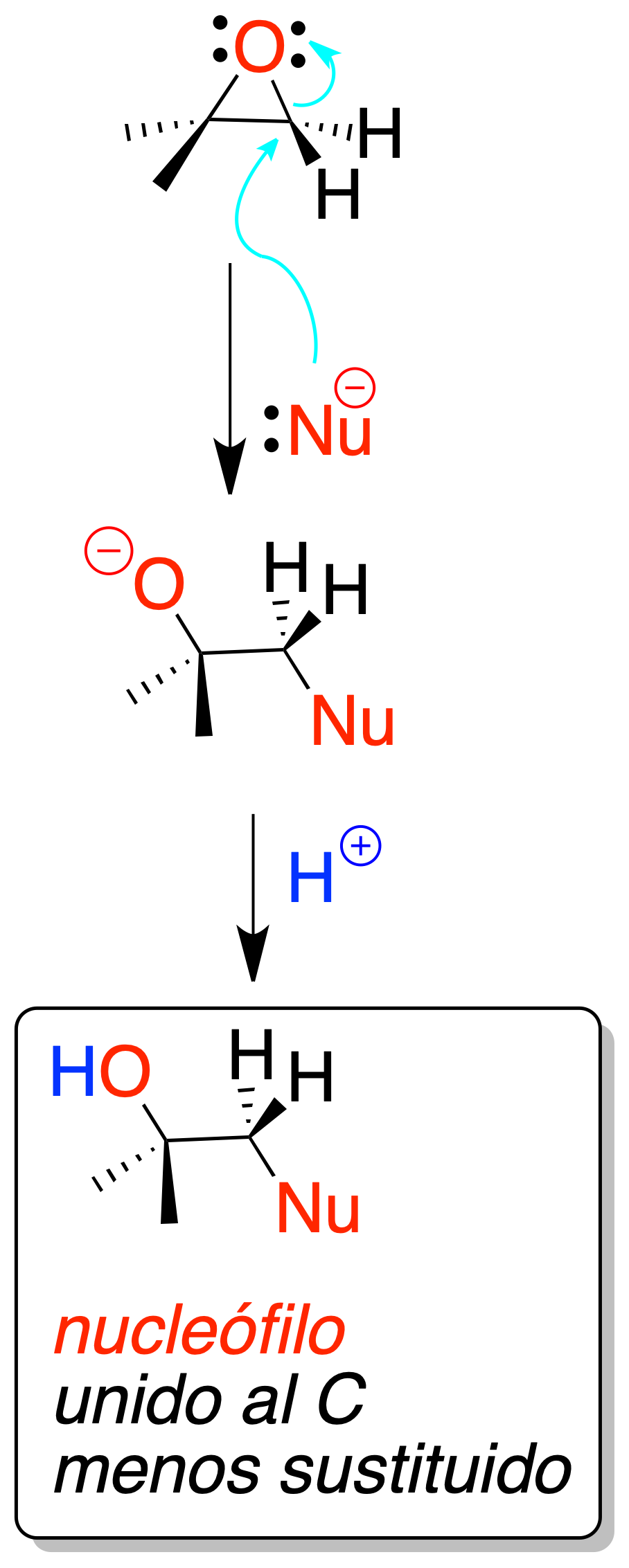
In the case of two diastereomeric epoxides, when treated with the same nucleophile under the same reaction conditions, two diastereomers are obtained (diastereospecific reaction). It is a very versatile procedure because a wide variety of different nucleophiles can be used.