Written by J.A Dobado | Last Updated on April 22, 2024
Radical halogenation
The halogenation of alkanes consists of the substitution of one or more hydrogens of a saturated hydrocarbon by a halogen atom or atoms.
It is a radical reaction with homolytic halogen cleavage initiated by appropriate radiation (hν) or by the presence of radical initiators (e.g. -O-O- peroxides).
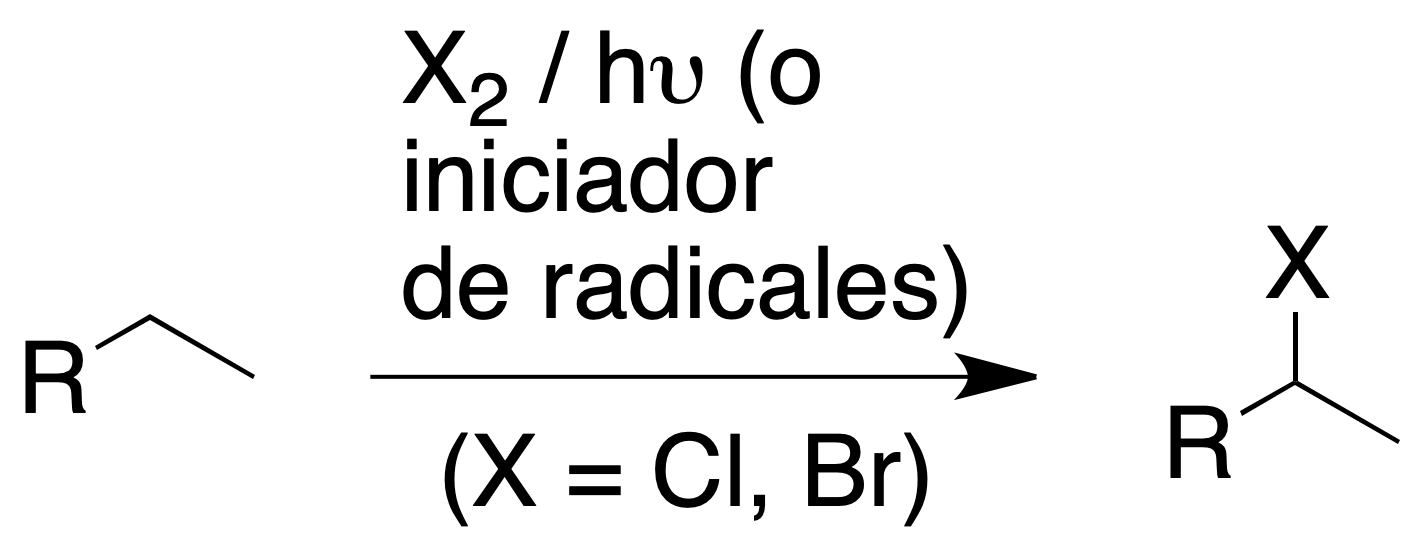
Only chlorination and bromination are of synthetic interest, both reactions being exothermic. Fluorination is uncontrollable (explosive process), while iodination has a very low reactivity.
Since halogenation is a radical reaction, it is very difficult to control and usually leads to mixtures of products with the halogen in the various positions of the alkane. The radical halogenation of alkanes and cycloalkanes depends on:
- The nature of the hydrocarbon: the reactivity of the different types of hydrogens in an alkane is not the same. The homolytic cleavage of a C-H bond can produce primary secondary or tertiary radicals which have different stability and are therefore not formed with the same ease. The order of stability of the different types of carbon radicals is as follows: tertiary > secondary > primary.
- The number of hydrogens of the same class (primary, secondary and tertiary): also conditions the final result (statistical factor).
- The halogen used: the reaction with chlorine is not very selective. In contrast, bromination has a high selectivity.
This reaction is particularly useful from the synthetic point of view when all the hydrogens of the molecule are equivalent (methane, cyclohexane, neopentane, etc.), because it gives a single reaction product (no mixtures).
Nevertheless, it is possible to estimate or predict the proportion of the various monohalogenation products by considering the approximate relative reactivities of the different types of radicals:
For chlorine: tertiary / secondary / primary is 5.1/3.6/1
For bromine: tertiary / secondary / primary is 990/99/1
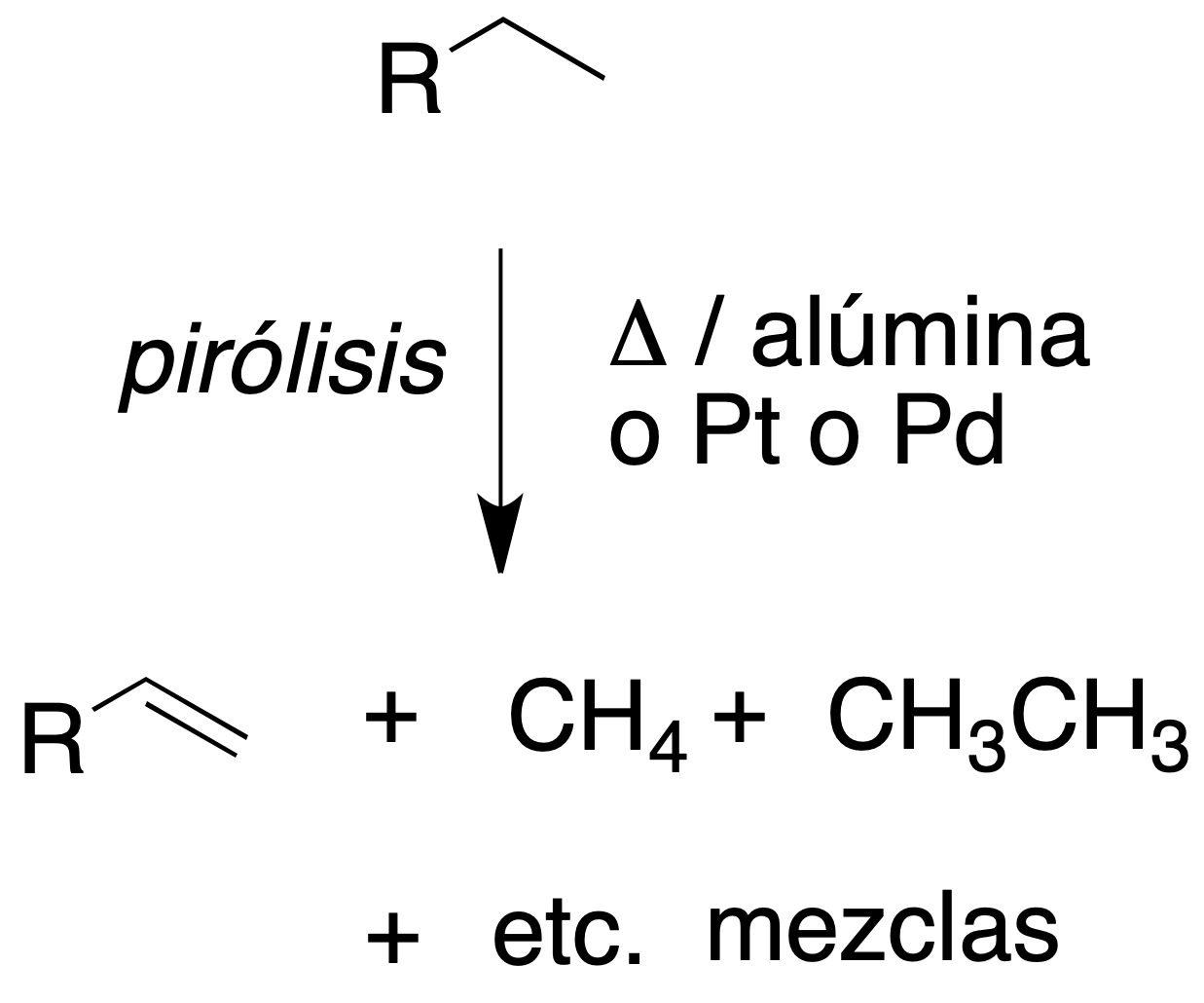
Pyrolysis
Pyrolysis or cracking consists of obtaining hydrocarbons of lower molecular mass from larger ones (usually by distillation of petroleum).
The reaction is carried out by heating the hydrocarbons in the absence of oxygen (thermal pyrolysis) or by heating over silica or alumina catalysts (catalytic pyrolysis).
The latter procedure is currently the most widely used, since it requires a lower temperature.
Alkanes are fragmented by breaking C-C and C-H bonds randomly, and free radicals with a lower number of carbons than the starting hydrocarbons are produced.
Smaller alkanes (with or without the carbon skeleton) and alkenes are usually obtained.
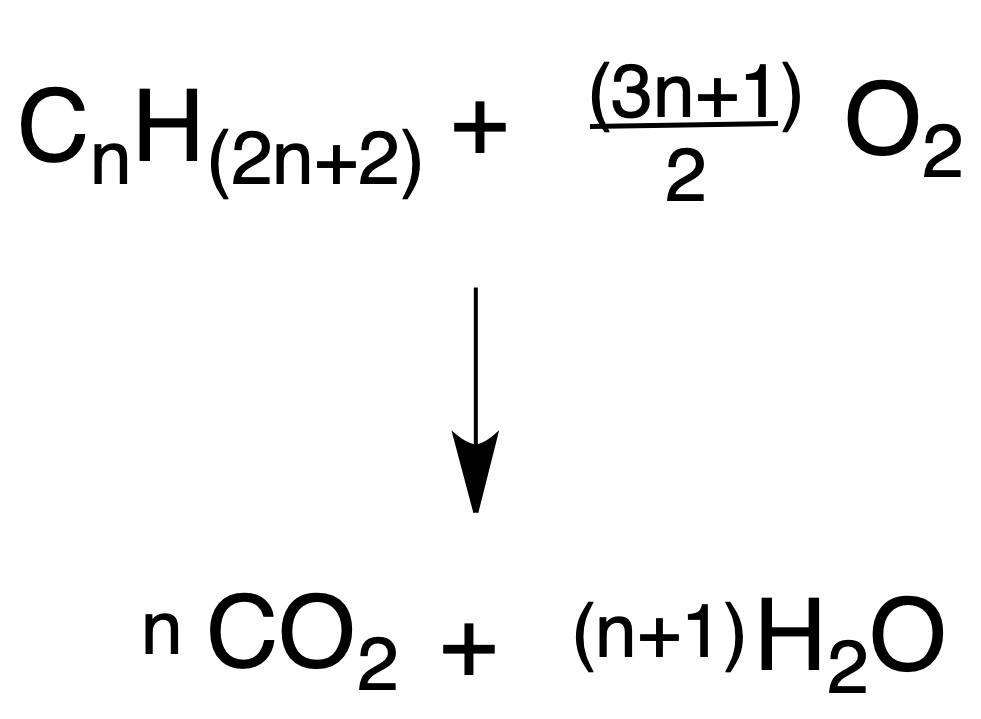
Combustion
The interest of this transformation lies in the large amount of energy that is released, and this is the basis for their use as fuels.
An important aspect of the reaction is the stoichiometry, because if there is not enough oxygen, highly toxic carbon monoxide (CO) is generated. For example:
CH3CH2CH3 + 4O2 → CO2 + 2CO + 4H2O
Oxidation intermediates (mainly aldehydes and carboxylic acids) can be formed when the amount of oxygen is low and/or the reaction temperature is not high enough.
This is one of the main causes of pollution generated by internal combustion engines in cities.