Written by J.A Dobado | Last Updated on April 22, 2024
What are dienes?
Dienes are compounds that have two double bonds (>C=C<).
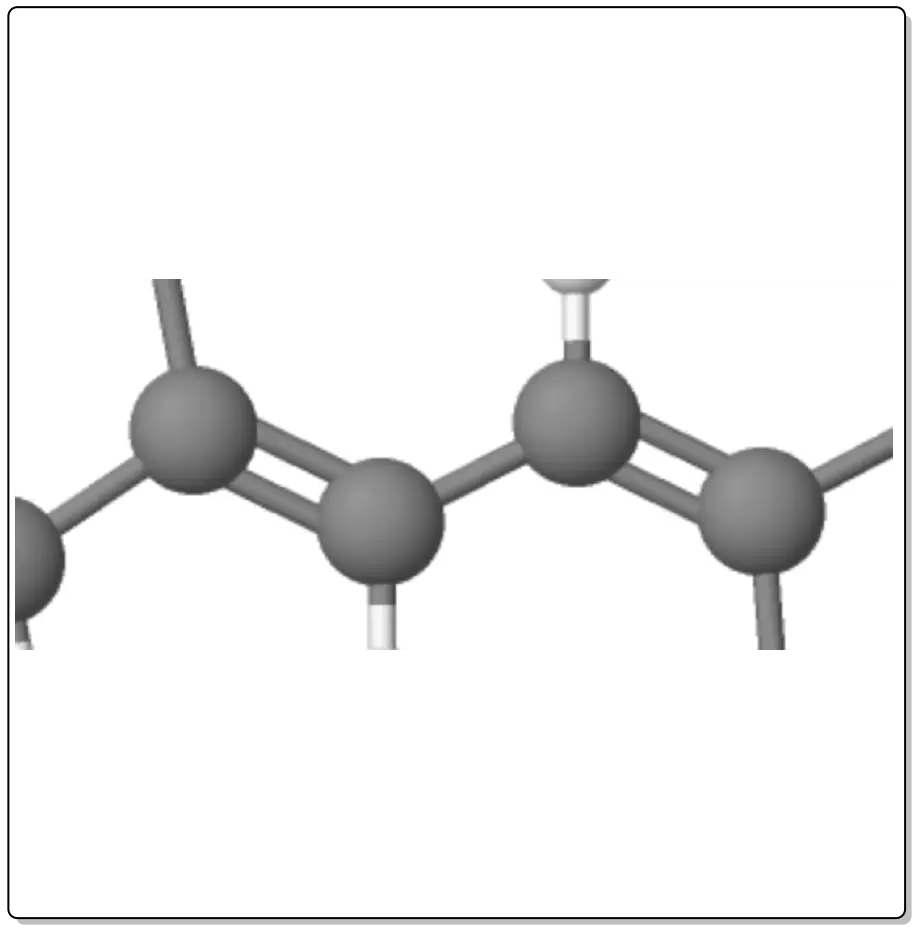
Depending on the relative arrangement of these double bonds, they have different characteristics. Thus we can have:
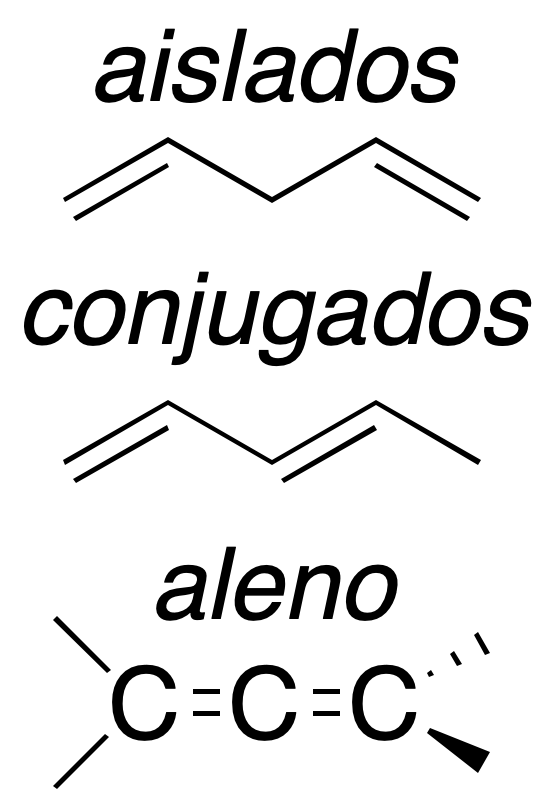
Isolated dienes
The two double bonds are separated by at least one carbon atom. They behave like conventional alkenes, so their reactivity is similar to that studied above.
Conjugated dienes
The double bonds are joined by a single bond. This arrangement gives the system extra stability and particular chemical properties as a result of mutual influence.
Cumulated dienes
They are called allenes (also cumulenes) and have a common carbon with sp hybridization. Their reactivity is more similar to alkynes than to olefins, due to the type of hybridization of the carbon common to the two double bonds. They are the most unstable of the group.
Electrophilic addition to conjugated dienes
In conjugated dienes the electrophilic addition of e.g. 1 mol XH has special features. The result of the reaction is temperature-dependent. As indicated in the scheme, the cause of such behavior is the resonance of the propenyl (allyl) cation produced after addition of the electrophile.
At low temperatures the 1,2 addition product is in the majority (H⊕ is added on the less substituted carbon and the nucleophile X⊖ on the more substituted one according to Markovnikov’s rule).
The formation of this compound is faster (kinetic control) than that of the 1,4-addition product. At higher temperature the proportion of the 1,4-addition product increases, and the thermodynamically more stable product is obtained (thermodynamic control).
As the speeds at which the two processes occur do not differ greatly, mixtures of the two products are normally obtained.
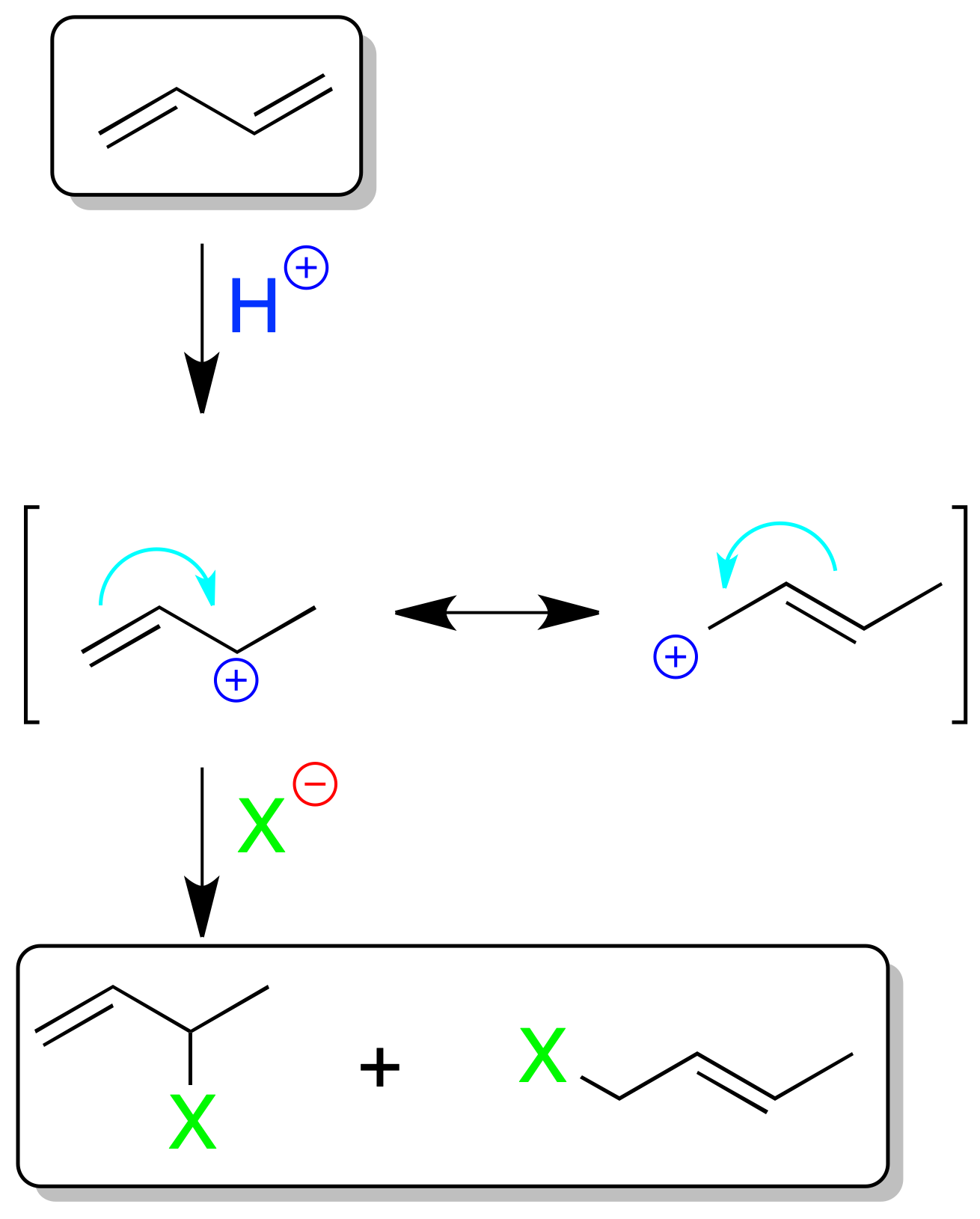
Similarly, bromination or chlorination of 1,3-dienes can give a mixture of the addition product 1,2 and 1,4, as in the previous reaction. Likewise, the concept of kinetic control and thermodynamic control applies in this case.
Diels-Alder reaction
Polymerization of dienes
Dienes can give polymers by polymerization processes involving a single double bond, as in the case of a conventional alkene, or by the intervention of both double bonds.
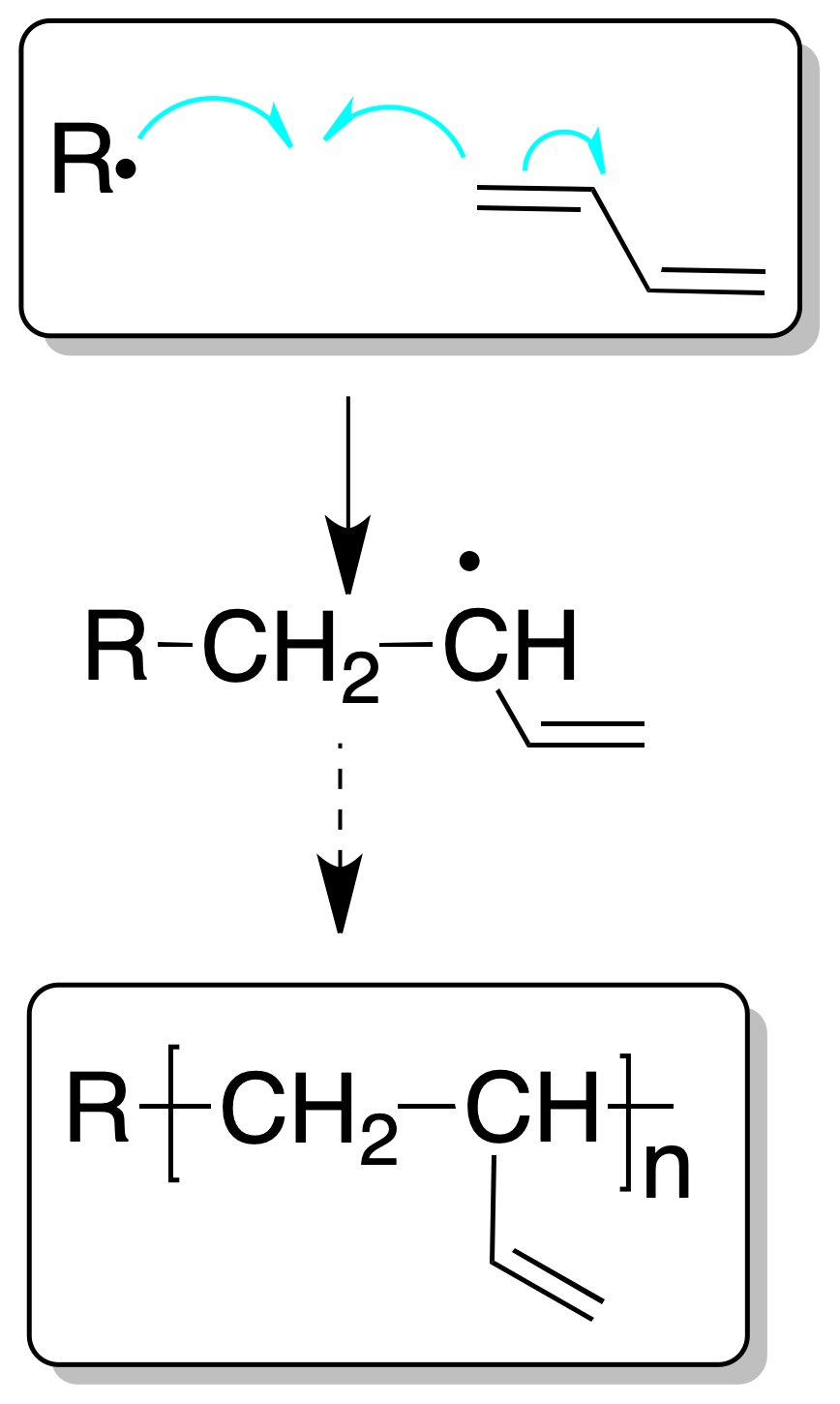
In the first case they are called 1,2 propagation processes and give rise to branched chains.
In the second case, it is called 1,4 propagation, and would give rise to linear chains.

Although the polymerization process, as in the case of simple alkenes, can be cationic, radical or anionic, the process using a radical mechanism is used on an industrial scale.