What is mass spectrometry (MS)?
Although it is included among the spectroscopic techniques, mass spectrometry (MS) is not one of these techniques because it does not use any radiation from the electromagnetic spectrum to irradiate the sample and observe the absorption of such radiation.
In EM, the sample is ionized (and therefore destroyed) using various procedures. Of all of them, the most widely used is the Electronic Impact technique (EM-IE) consisting of bombarding the sample (previously vaporized with high vacuum and a heat source) with a high-speed electron current. Therefore, the molecule in turn loses some electrons and fragments giving different ions, radicals and neutral molecules.
The ions (charged molecules or fragments), and only them, are conducted by an ion accelerator into a curved analyzer tube with a strong magnetic field. In this way, they are directed to a collector/analyzer on which their impacts are collected. This is a function of their charge/mass ratio:
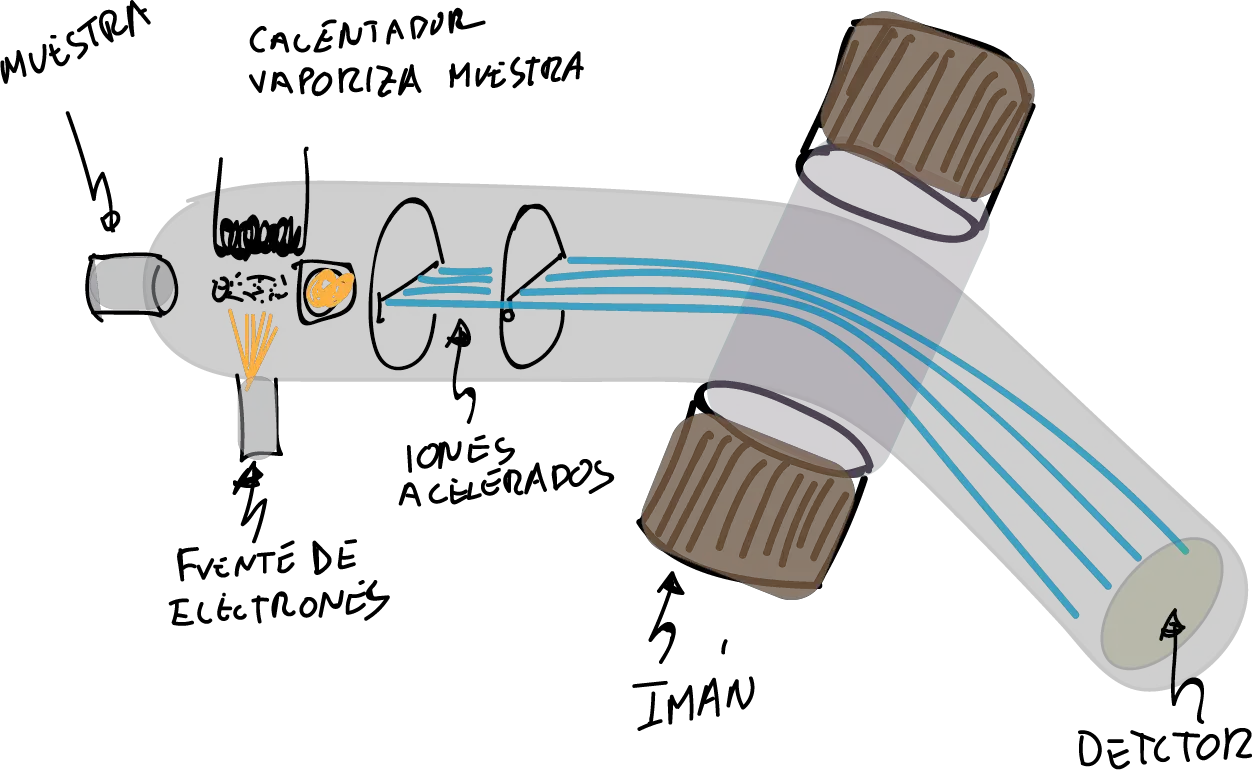
Subsequently, these impacts are transformed into a mass spectrum as shown below:
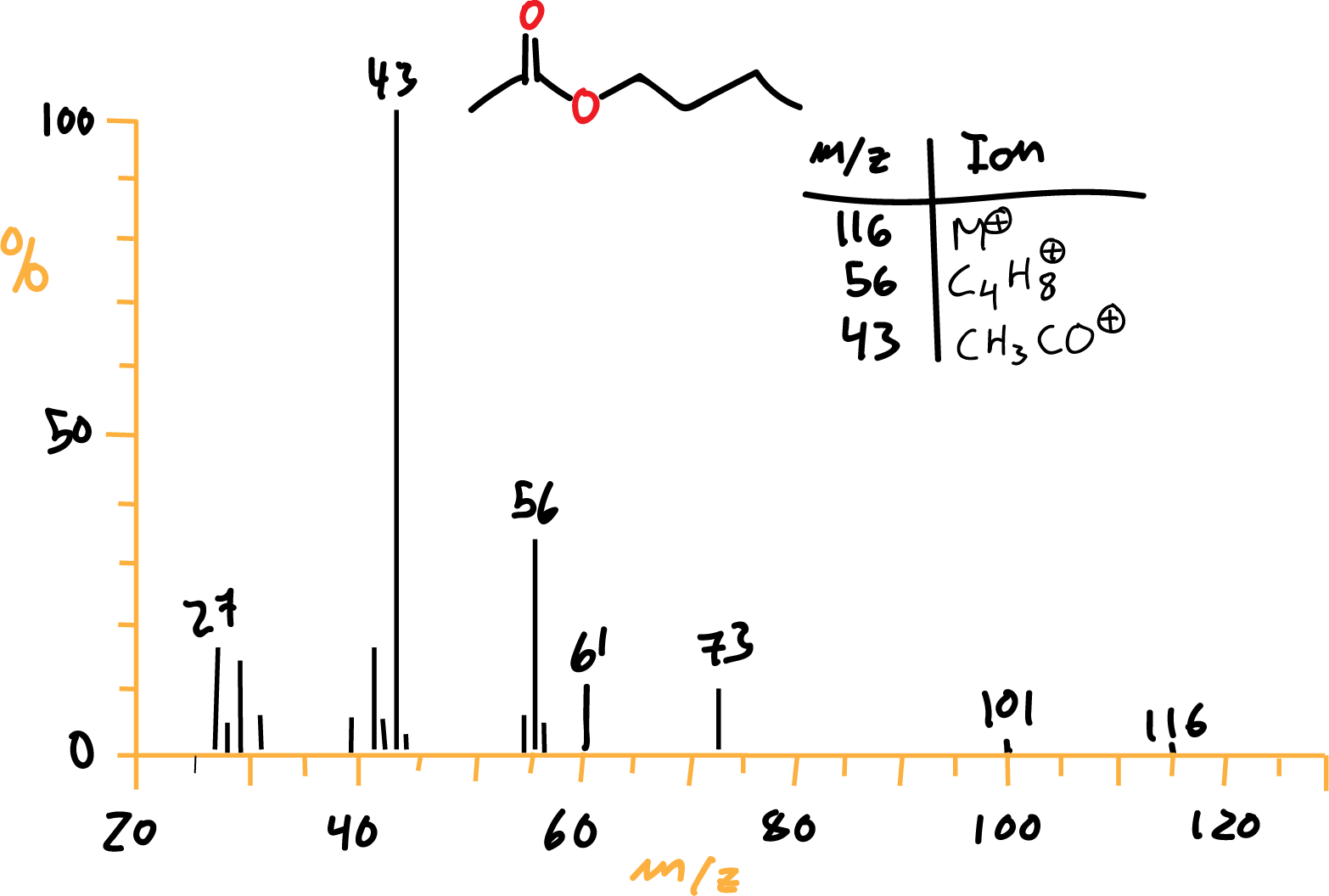
In which the intensity of the peaks indicates the relative amount of ions of this charge/mass ratio. The separation of the different ions is based on:
Where H is the magnetic field strength, r the deflection radius of the analyzer tube and V the acceleration potential used.
This expression can be deduced from the expressions relating, on the one hand, the kinetic energy of the ions:
and on the other, the motion of a charged particle in a magnetic field:
| Curiosities! “the ion velocity is usually about 100 km/s, the deflection radius (r) about 35-50 cm and the analyzer tube is usually a spherical sector of about one meter in length.“ |
Metastable ions
The first issue that arises from this approach is that we must think that for an ion to be observed it must exist at least for the time it takes to travel through the analyzer (stable ions, on the order of 10 microseconds). Therefore, any ion with a half-life of less than 1 microsecond will not leave the ionization chamber (unstable ions). However, those ions with a half-life between 1 and 10 microseconds will decay during the path and arrive at the collector/detector with a mass different from the starting mass (meta-stable ions).
Suppose that an A+ ion comes out of the ionization chamber and decays along the way giving a new E+ ion and losing a neutral molecule N (or an uncharged radical):
Initially:
2·e·V = mTo·v2,
but in the deflector tube:
e·r·H = mE·v
Rearranging we will obtain:
m* = mE2/mTo = r2·H2/2V
i.e., an ion of apparent mass will appear:
m* = mE2/mTo
As special characteristics of these metastable ions we will highlight:
- The fact that they are usually broad peaks (not sharp as observed in normal spectra).
- Moreover, that their apparent mass is less than that of the two ions that give rise to their appearance, less than mEand less than mA.
- Finally, the appearance of one of these peaks in the spectrum indicates unequivocally the presence of a fragmentation process between the two ions involved.
MS-EI of organic compounds
Having seen the fragmentation modes, we will review the most common fragmentations of the different functional groups.
Saturated hydrocarbons
In them, the molecular peak usually appears although sometimes with a low intensity. Its spectrum presents a set of peaks separated in 14 mass units. The most intense peaks are usually those corresponding to C3 and C4 (m/e 43 and 57, respectively).
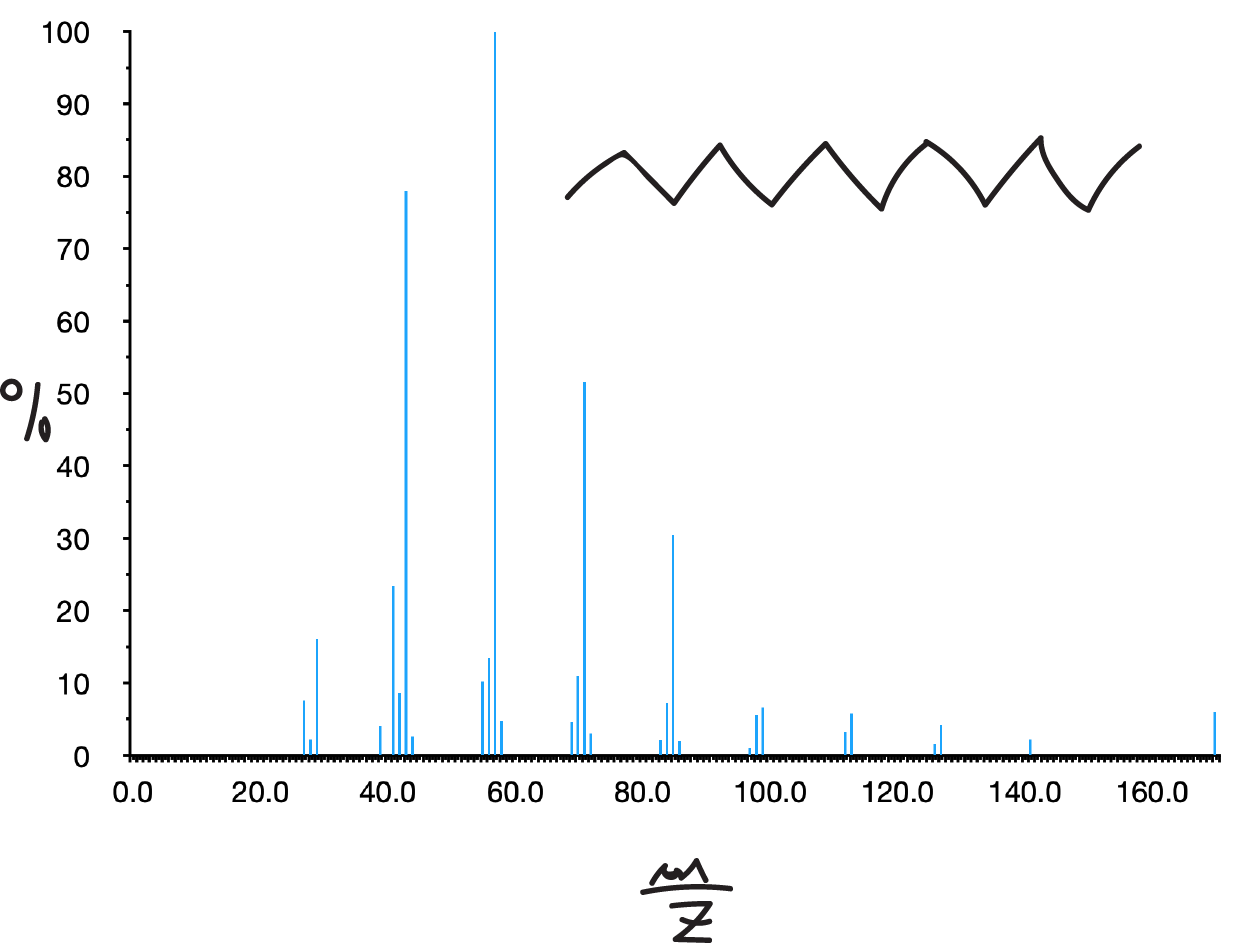
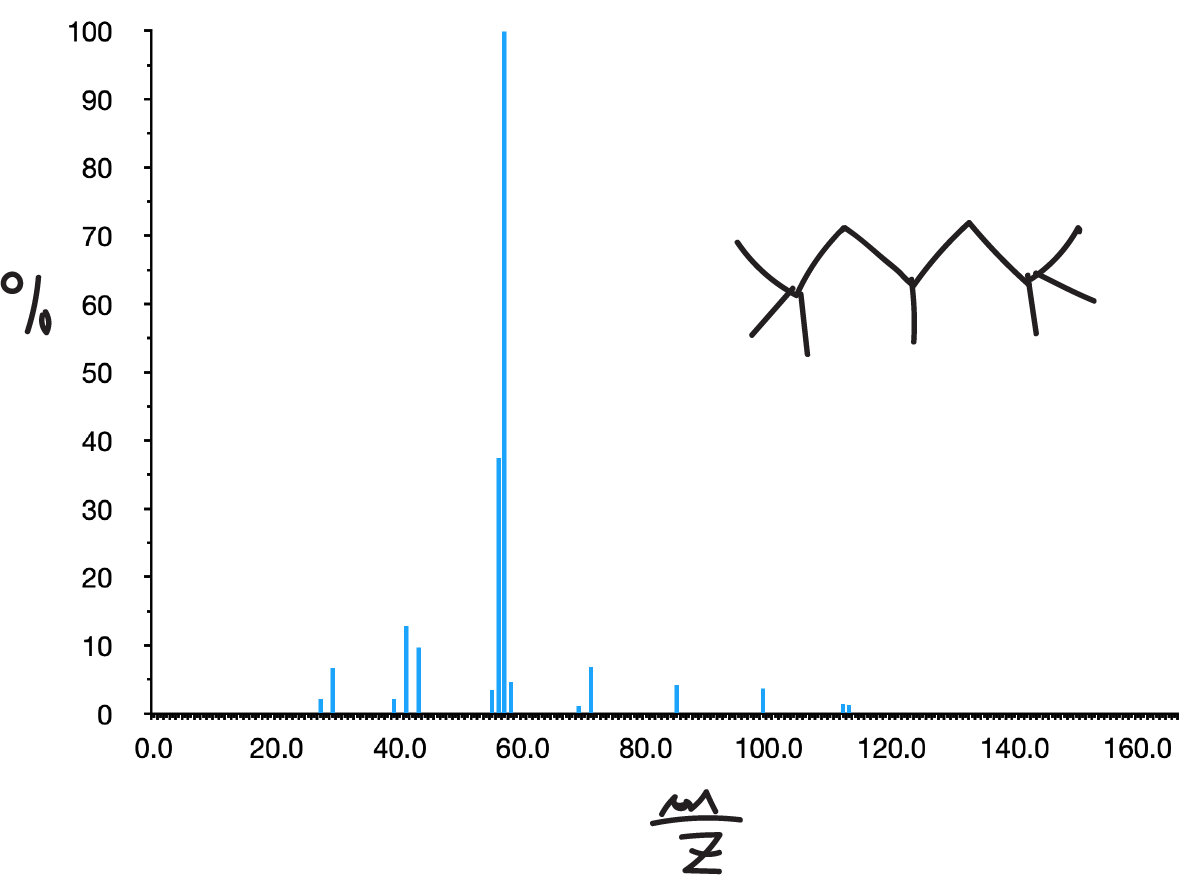
The presence of branching leads to breakage at the branching points (1st simple fragmentation rule) increasing the intensities of the secondary ions formed at these breaks.
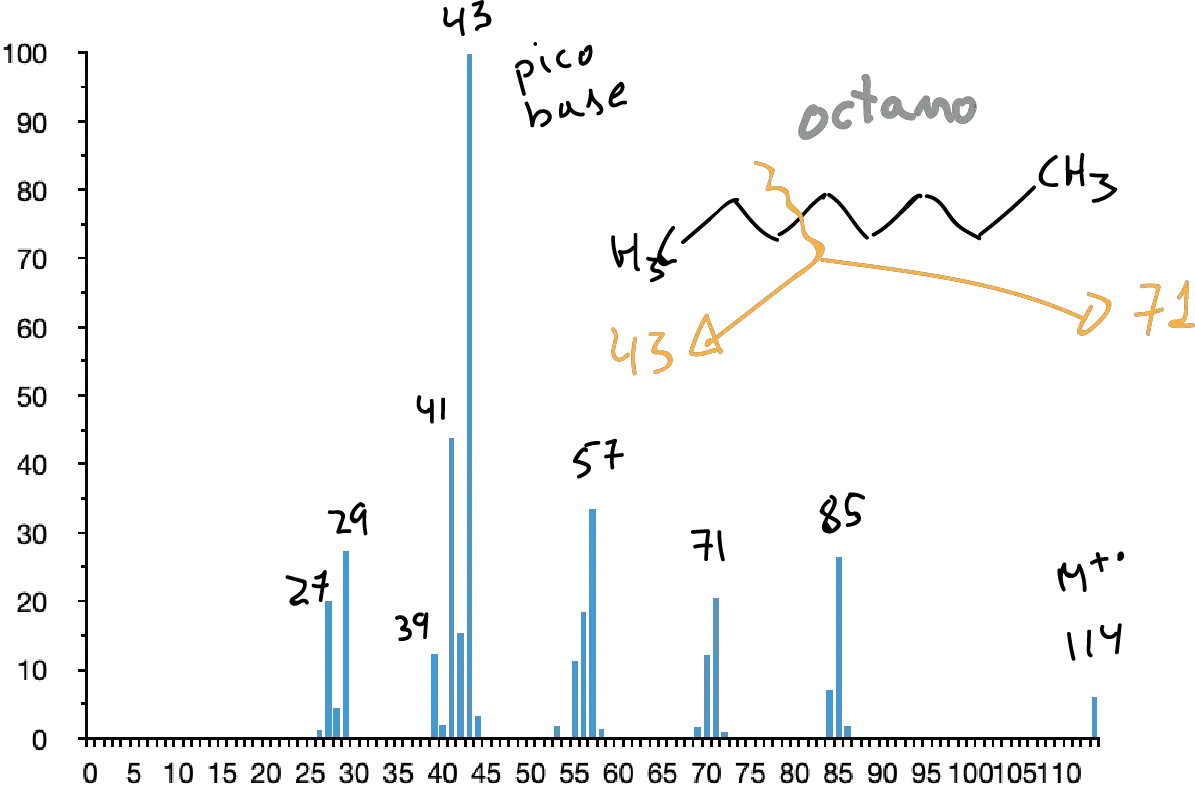
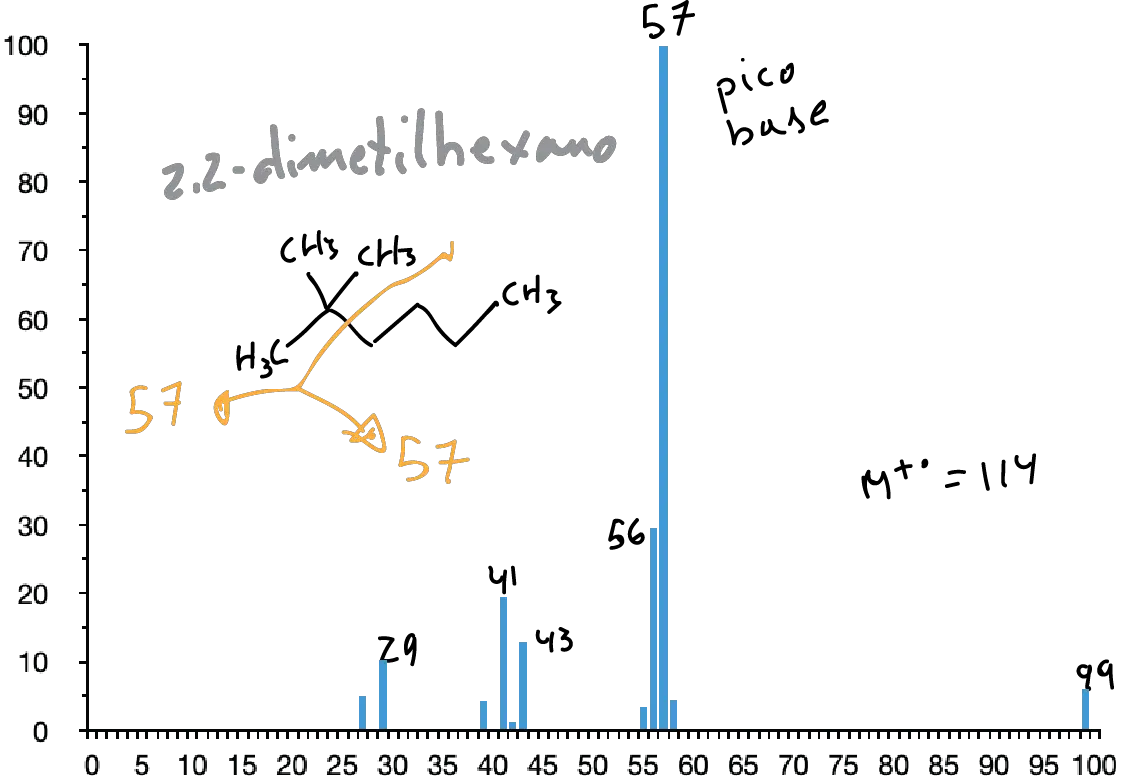
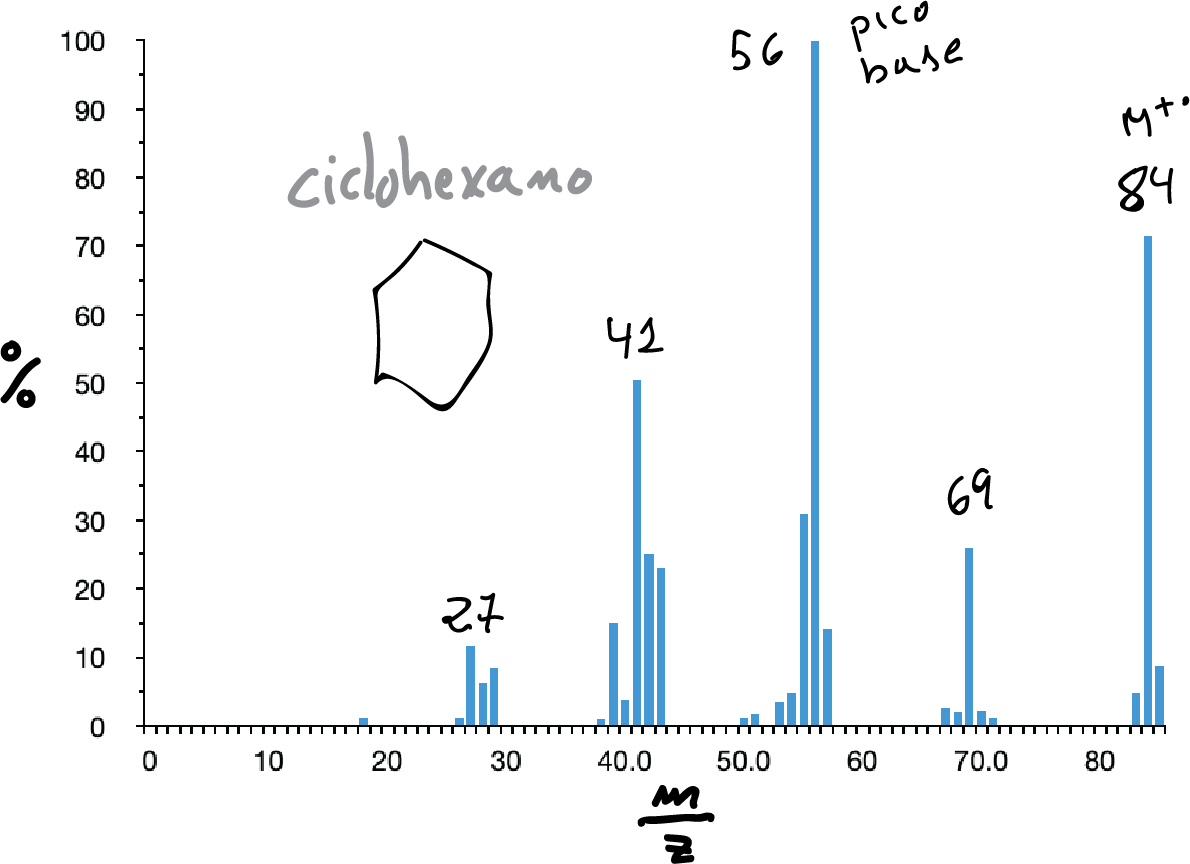
Saturated alicyclic saturated hydrocarbons usually have a strong molecular peak. They are usually complex spectra in which the loss of ethylene (M-28) and the loss of the side chain (if present) are predominant.
Unsaturated hydrocarbons
They usually present the molecular ion peak, apparently formed by the loss of a π electron. The most representative peaks usually correspond to the formation of allylic cations as a consequence of the breaking of bonds in the allylic position with respect to the double bond (2nd Fragmentation Rule).
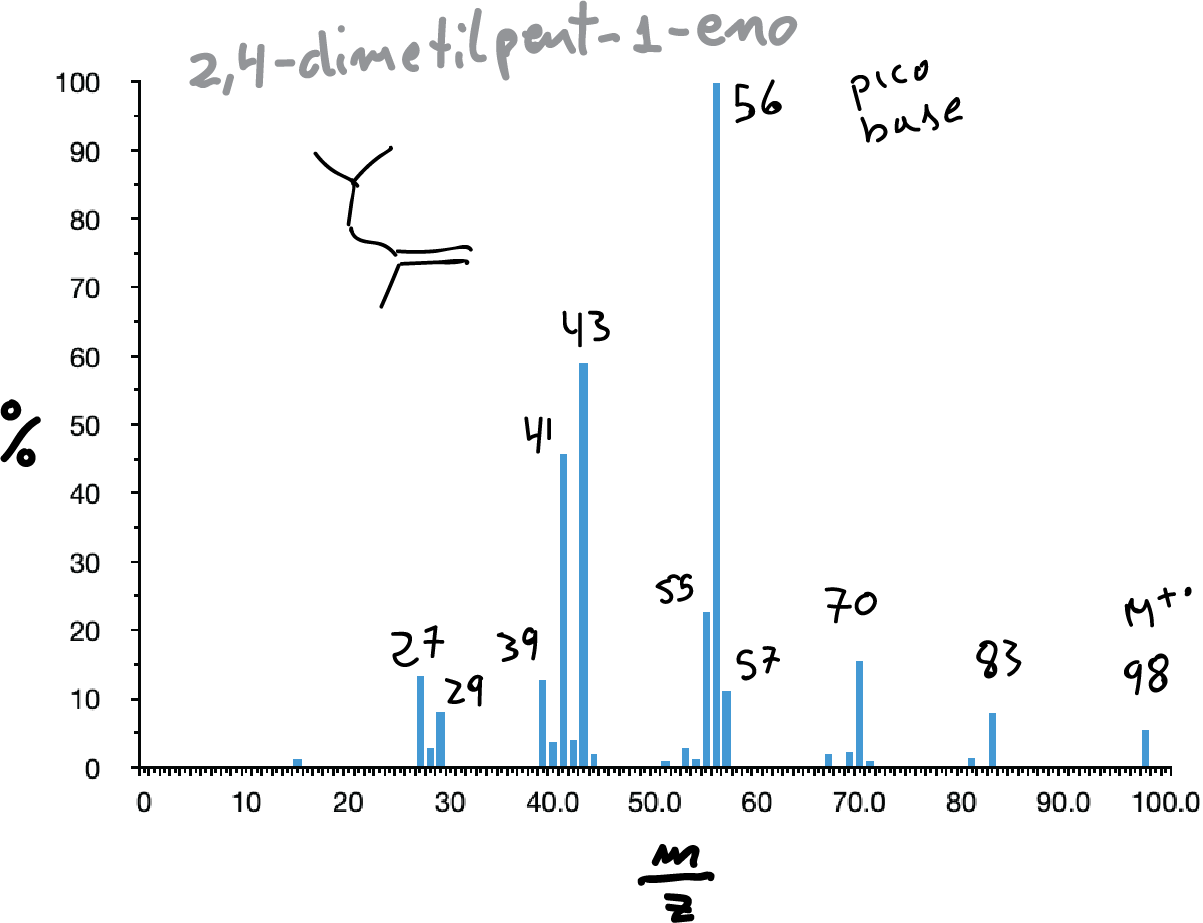
However, the possibility of transpositions or regroupings and also the possibility of McLafferty rearrangement must be taken into account.
The formation of ions corresponding to the Retro-Diels-Alder is usually observed in cyclic hydrocarbons.
Aromatic hydrocarbons
Aromatic hydrocarbons usually show a strong molecular ion peak. If they are branched (alkylbenzenes) the most characteristic breakage usually corresponds to the formation of tropylium ion (C7H7, m/z = 91) or substituted tropylium ions, usually accompanied by a peak at m/z = 65 due to the loss of acetylene from it. If the chain is long enough, McLafferty transposition peaks are also observed (m/z = 92).
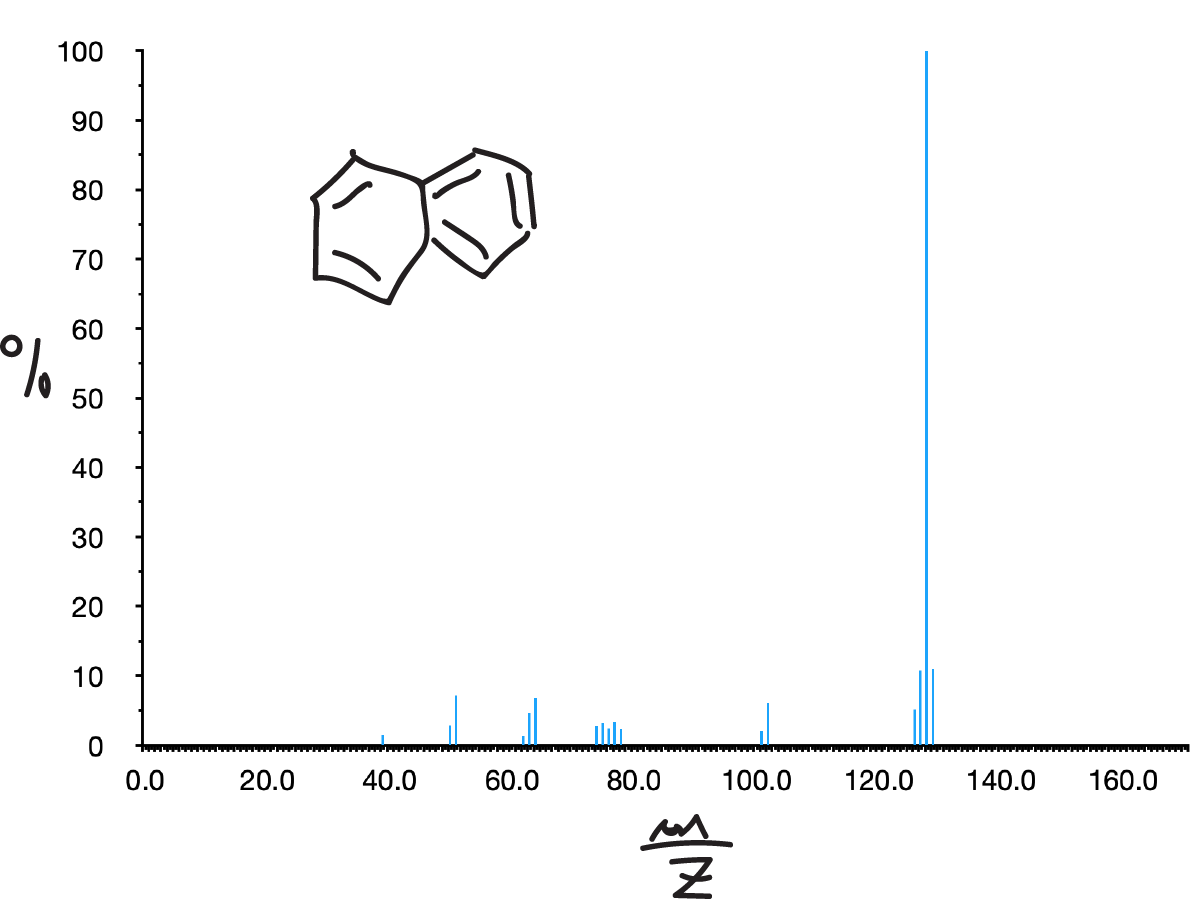
Halides
While fluorine and iodine are monoisotopic, chlorine and bromine are not, so their presence is evidenced by the appearance of two peaks corresponding to the molecular ion of masses M and M+2, in the case of chlorine the latter with an intensity 1/3 of M and in the case of bromine practically equal in intensity.
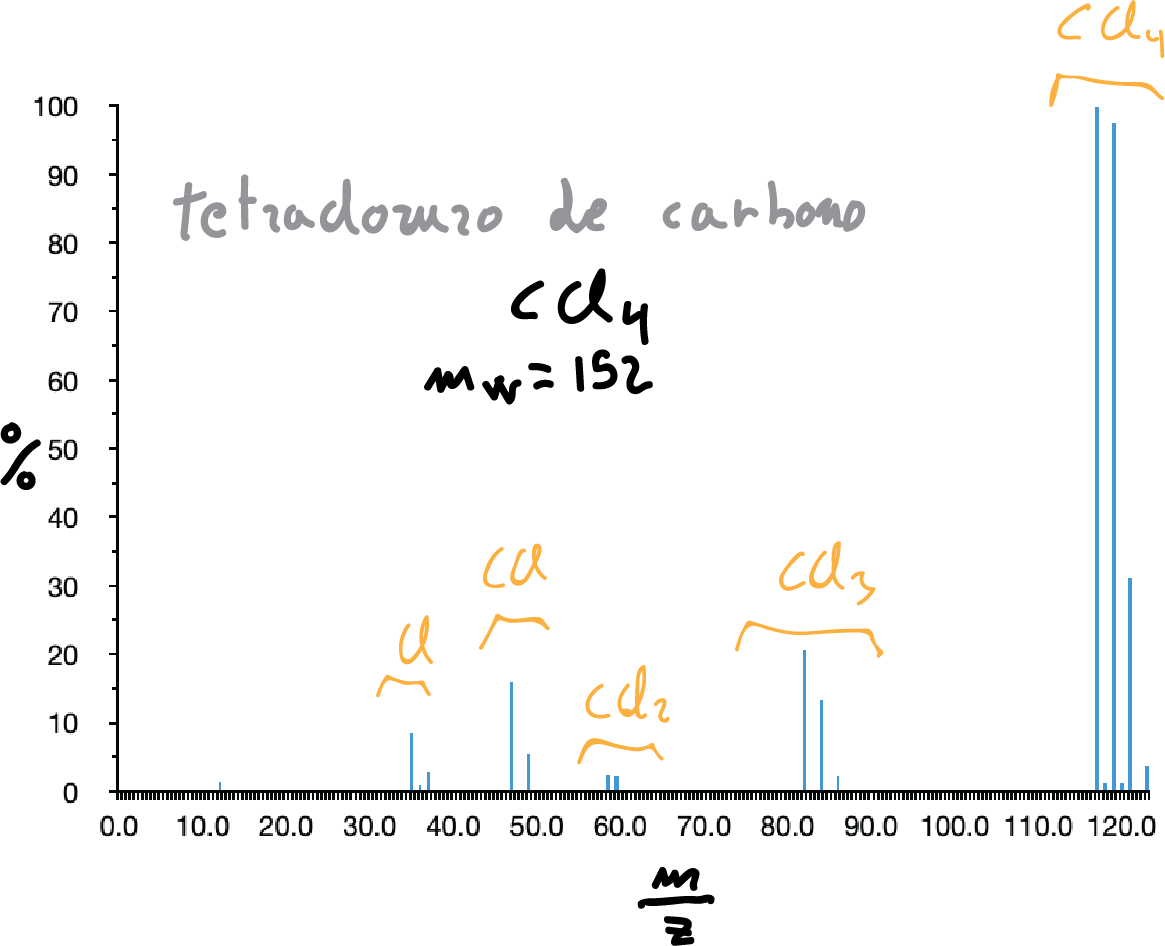
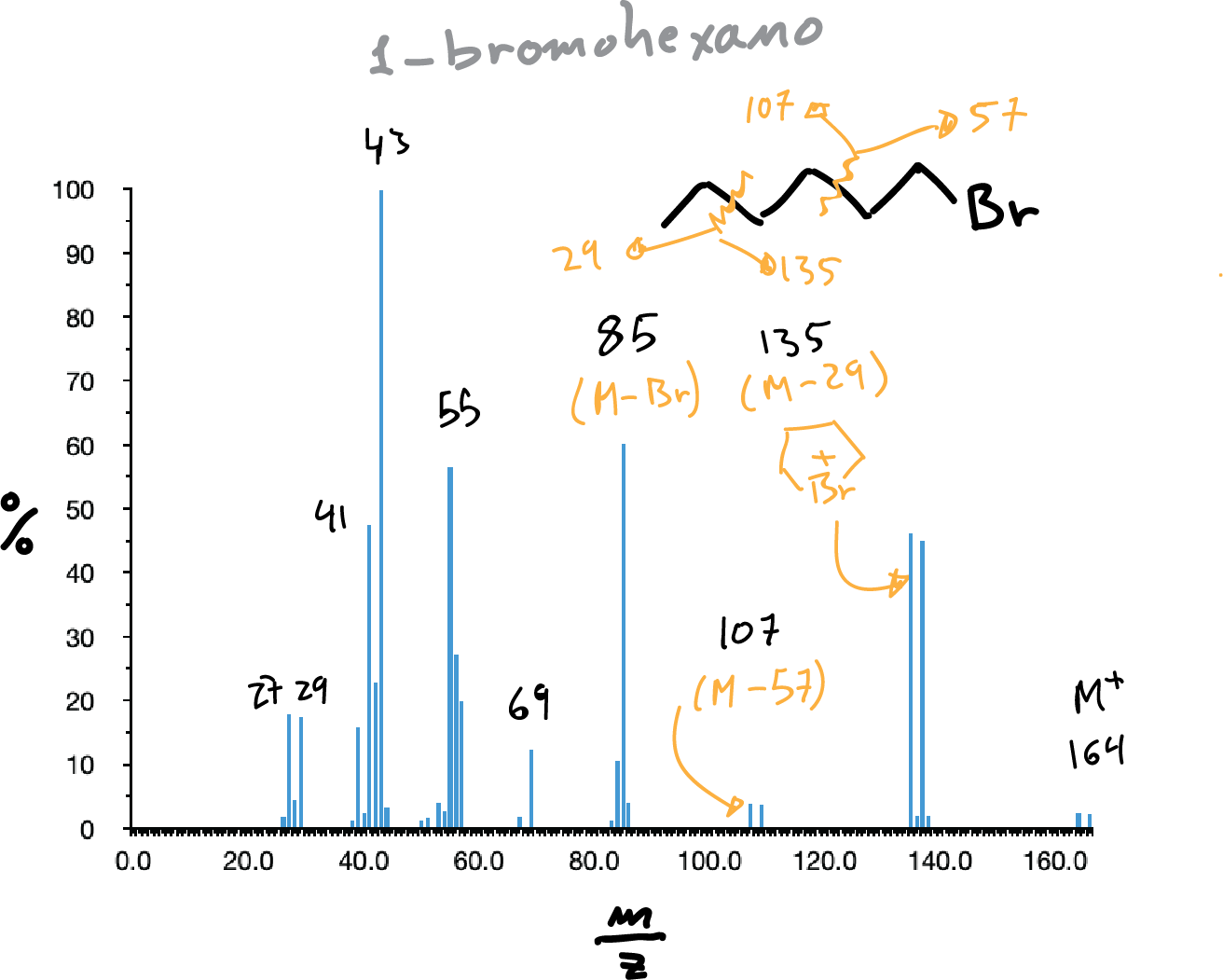
The most abundant fragment usually corresponds to the breaking of the C-X bond giving rise to C+ (3rd simple fragmentation rule). Loss of HCl and formation of the alkene is frequent.
Also, cyclic bromonium unions often appear when a 5-membered ring can be formed (see in the figure below the peak at m/z = 135).
Alcohols
Primary and secondary alcohols usually have a weak molecular ion peak, tertiary alcohols usually do not.
The most frequent fragmentation processes are: dehydration (M-18) and the breaking of a bond in the carbon that supports the -OH group.
The latter gives rise to ions of the type: R2C+-OH stabilized by resonance by oxygen: CH2OH of (m/e 31) in the case of primary alcohols) (3rd simple fragmentation rule).
In addition, benzyl alcohols usually give an intense molecular ion peak and can give M-1 (hydroxypropyl) and/or M-18 by ortho effect.
fig-8
Phenols
They usually give an intense molecular ion peak and a characteristic peak at M-28 (loss of CO).
fig-9
Ethers
They have peaks similar to those of alcohols. The two most frequent fragmentation processes are usually:
- C-O bond breaking with the charge remaining on the alkyl radical (except in the case of aromatics where the charge usually remains on both the aromatic ring and the phenoxide).
fig-10
- C-COH bond breaking, giving rise to ions of the type: R2C+-OR stabilized by resonance by oxygen: CH2OR (31, 45, 59, …).
Aldehydes
Aliphatic aldehydes have weak molecular ion peaks. Thus, they usually give the alpha bond breakage with respect to the carbonyl group: peaks M-1 (characteristic of aldehydes) and 29 (CHO).
If they possess H in gamma position they can give McLafferty rearrangement: m/e 44 (CH2=CHOH) and M-44. (3rd and 4th rules of simple fragmentation).
fig-11
Aromatics aldehydes present intense molecular ions and also intense M-1, which usually gives the loss of CO to produce phenyl ions.
Ketones
Ketones have a molecular ion that is usually intense. Therefore, they fragment in a manner analogous to aldehydes, with the acyl ion (RCO) generally predominating as a result of the loss of the bulkier alkyl residue. They usually have a base peak at m/e 43 (CH3CO).
When there are hydrogens in gamma the peaks of the McLafferty rearrangement predominate giving up to 3 possible transpositions ending with a peak at m/e 58 (CH3COH=CH2).
fig-12
Aromatics usually have the benzoyl ion m/e 105 as their base peak.
fig-13
Acids
If possible they give McLafferty rearrangement, so in aliphatics the base peak is usually at m/e 60 (CH2=C(OH)2). They usually have a peak at m/e 45 corresponding to COOH.
fig-14
In aromatics the M-17 peak corresponding to the acyl ion is usually very intense. Their methyl esters are usually used to study them by MS.
Esters
Methyl esters usually give acyl (RCO) and carboxymethyl (OCOCH3, m/e 59) ions as key ions.
fig-15
If long-chain, the McLafferty rearrangement product (m/e 74: CH3COH=CH2) is obtained. Ethyl esters and higher esters usually give the R-COOH ion due to such rearrangement.
fig-16
Those of aromatic acids usually give significant acyl ions (ArCO) due to loss of the alkoxyl radical and McLafferty rearrangement. Benzyls usually give a peak at M-42 due to loss of ketene (CH2=C=O).
fig-17
Amines
Aliphatic amines do not usually have a molecular ion. The most intense peak usually corresponds to the loss of an alkyl group in beta with respect to nitrogen (R2C=N+R’2), which in the case of primary amines corresponds to m/e 30 (CH2=NH2).
fig-18
Aromatics usually give an intense molecular ion peak that is usually accompanied by a moderate M-1.
fig-19
Amides
The molecular peak is usually observed. The presence of a peak at m/e 44 (NH2=C=O) is indicative of primary amides. Amides usually give McLafferty rearrangements.
fig-20
Nitriles
In nitriles, the base peak is generally due to a McLafferty rearrangement and corresponds to the CH2=C=NH ion (m/e 41).
fig-21
Nitro compounds
Nitrated compounds typically give losses of 30 (NO) and 46 (NO2).
fig-22
Sulfur compounds
Thiols and sulfides behave like alcohols and ethers, except that they have more intense molecular ions than alcohols and ethers. Disulfides usually show olefin losses: peak at m/e 66 corresponding to HSSH.
fig-23
Factors influencing the abundance of the ions
The most favorable mechanisms are determined by several forces:
- Reactions leading to more stable products both ionic and neutral. If a heteroatom (N, S, O) is present, the ions are often stabilized by formation of a “new bond” with the heteroatom.
- The reactivities in the ion have their parallel in the known reactions.
- Steric factors also play a role.
fig-24
- Resonance effects:
fig-25
- The inductive effect: (+I) of the alkyl groups (hyperconjugation) and (-I) according to the series: Cl > Br, O, S > I > I >> N
fig-26
fig-27
- The polarizability of the links retransmits along the links.
Molecular ion
As indicated above, when a molecule is introduced into the ionization chamber and bombarded with a stream of electrons it undergoes ionization. That is, the molecule loses an electron resulting in the formation of a radical-ion.
fig-28
Logically, the electron that is lost must be the one with the lowest ionization potential. Thus, structures like those shown in the figure will be obtained.
fig-29
For example:
fig-30
Molecular ion properties
Among others, the molecular ion must possess the following characteristics:
- To be the ion with the highest mass of those appearing in the spectrum.
- Contain all the elements present in its fragments.
- Correspond to the ion with the lowest ionization potential.
- Its m/e ratio must be even when it has no nitrogen or has an even number of N. For the same reason, it must be odd when it has an odd number of nitrogen atoms.
- The mass differences between this molecular ion and the fragments appearing in the spectrum must be chemically logical.
- Their relative abundance depends largely on the chemical nature of the product.
Relationship between intensity and main group
Therefore, there is a series that relates, in a qualitative and approximate way, the intensity of that ion with the main group present in the molecule.
Ordenadas de mayor a menor sería:
- aromatic compounds > conjugated olefins > alicyclic compounds > sulfides > linear hydrocarbons > mercaptans > ketones > amines > esters > ethers > carboxylic acids > branched hydrocarbons > alcohols
- The molecular ion can be used for the determination of the molecular formula of the substance.
- When a high resolution mass spectrum (HR-MS) is obtained, it is possible to distinguish between formulas of equal mass. This is because the elements have an atomic weight that makes it possible to distinguish between them.
In Table 1, some mass differences of atomic combinations for the peak value 43 are shown.
| Atom combination | Exact mass |
| CHNO | 43.0058 |
| C2H3O | 43.0184 |
| CH3N2 | 43.0269 |
| C2H5N | 43.0421 |
| C3H7 | 43.0547 |
Isotopic distribution
Furthermore, Table 2 shows the cause of these differences, which is none other than the exact mass of the elements due to their isotopic distribution.
| Element (Atomic weight) | Isotopes (relative abundance %) | Atomic mass |
| Hydrogen (1.00794) | 1H (100) 2H (0.015) | 1.00783 2.01410 |
| Carbon (12.01115) | 12C (100) 13C (1.12) | 12.00000 13.00336 |
| Nitrogen (14.0067) | 14N (100) 15N (0.366) | 14.0031 15.0001 |
| Oxygen (15.9994) | 16O (100) 17O (0.037) 18O (0.240) | 15.9949 16.9991 17.9992 |
| Fluorine (18.9984) | 19F (100) | 18.9984 |
| Silicon (28.0855) | 28Si (100) 29Si (5.110) 30Si (3.385) | 27.9769 28.97.65 29.9738 |
| Phosphorus (30.9738) | 31P (100) | 30.9738 |
| Sulfur (32.066) | 32S (100) 33S (0.789) 34S (4.438) 36S (0.018) | 31.9721 32.9715 33.9669 35.9677 |
| Chlorine (35.4527) | 35Cl (100) 37Cl (32.399) | 34.9689 36.9659 |
| Bromime (79.9094) | 79Br (100) 81Br (97.940) | 78.9183 80.9163 |
| Iodine (126.9045) | 127I (100) | 126.9045 |
Molecular ion fragmentations and transpositions
We can consider that there are three fundamental types of molecular ion fragmentations depending on the number of bonds that are broken:
Breaking of single bond (σ)
They always produce a cation and a radical, known as simple fragmentation:
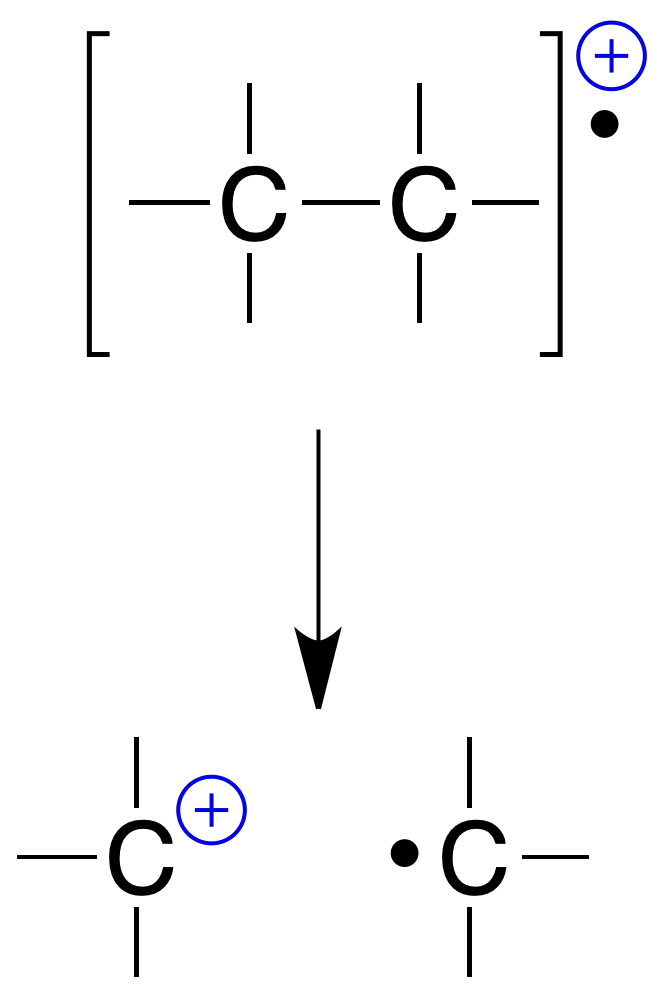
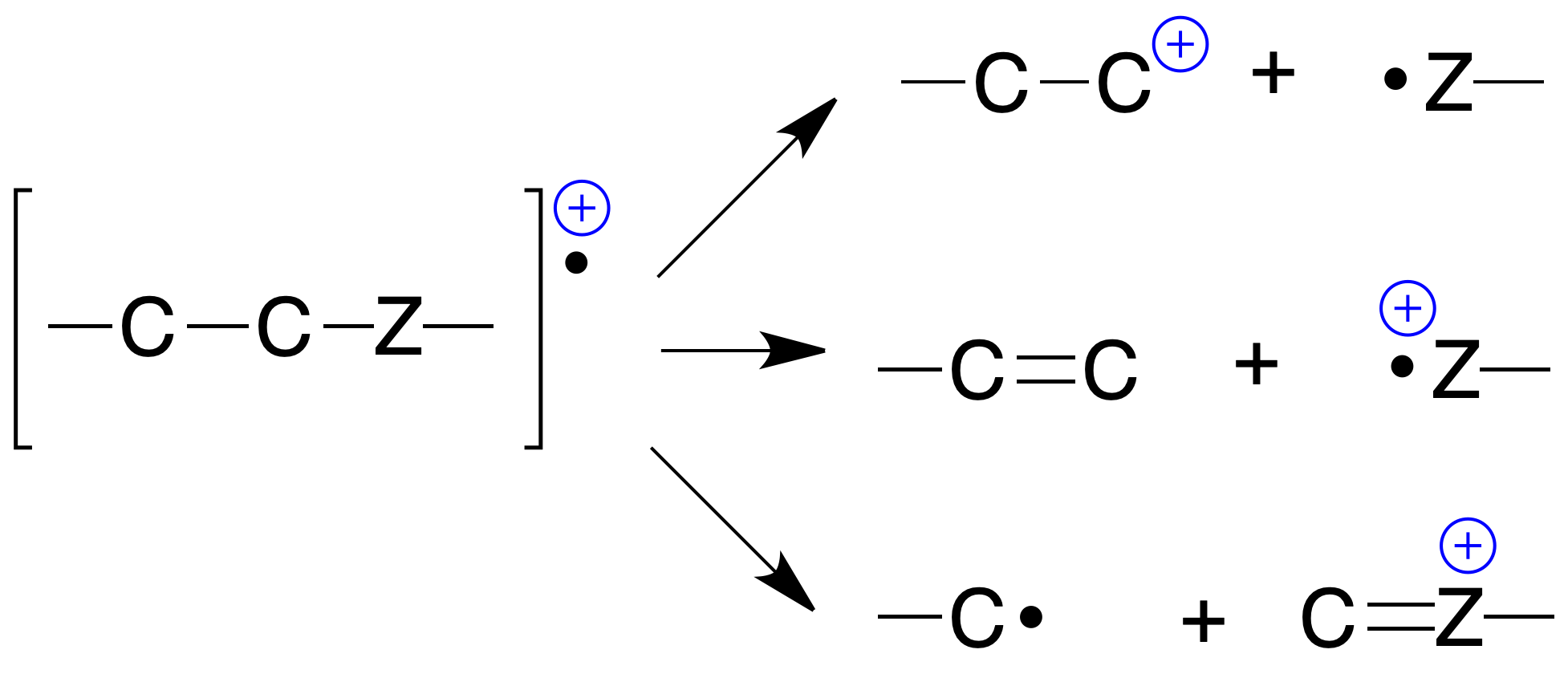
Simultaneous breaking of two single bonds
This breaking normally produces the elimination of neutral molecules. This type also includes transpositions or regroupings through cyclic states such as retro Diels-Alder and McLafferty rearrangement:
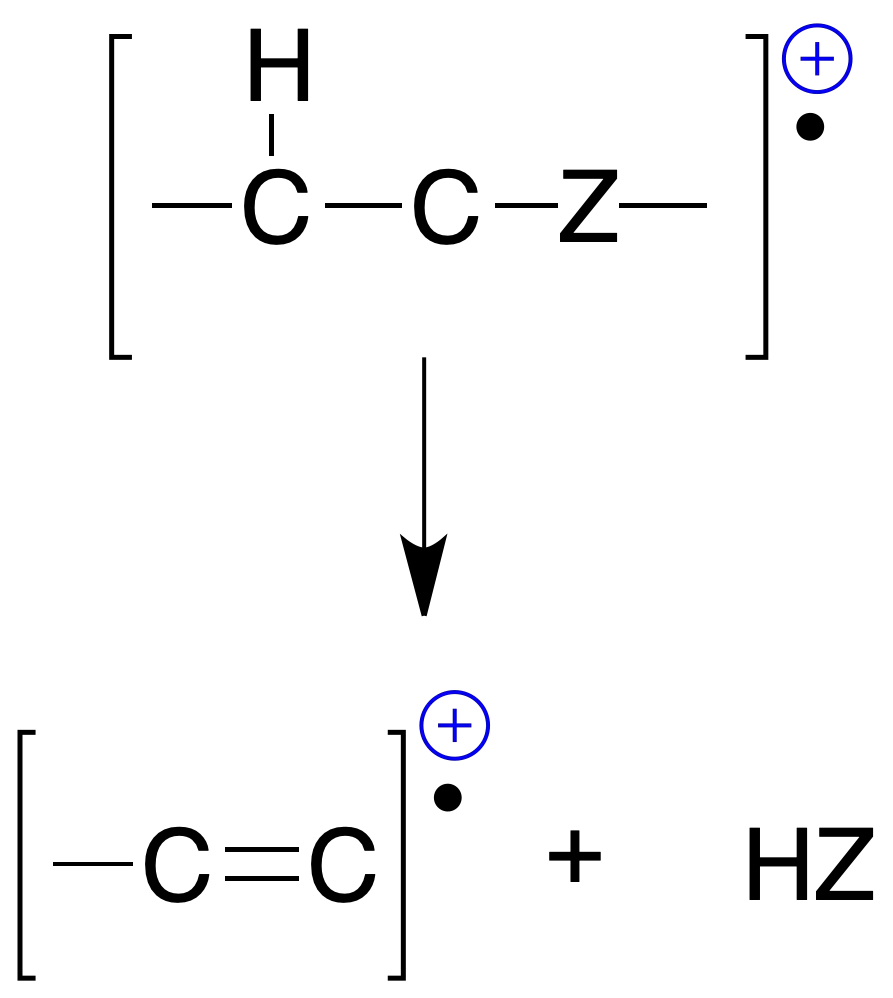
In addition, more complex transpositions involve hydrogen radical transfers and eliminations between non-neighboring atoms.
Depending on the type of bond breakage, single fragmentation can be homolytic or heterolytic. In both cases, resulting with the formation of a radical (which is not detected in the mass spectrum) and a cation:
fig-33

A characteristic feature of this simple fragmentation is that if the molecular ion does not contain nitrogen (its mass is even), the fragments have an odd mass.
In turn, the cations formed in this fragmentation can undergo further fragmentation either by homolytic (producing radicals and radical ions) or heterolytic (producing neutral molecules and another cation).
fig-34

Fragmentation of cyclic molecules
A somewhat special case is that of cyclic molecules, which when a ring bond is broken, a new radical ion is obtained, homologous to the molecular ion, but linear.

These radical ions subsequently undergo further fragmentations and/or transpositions, giving rise to cations of even or odd mass, respectively.
fig-36


Most common fragmentations
Table 3 includes the masses of the most characteristic and frequent fragments that appear in the mass spectra. It should be noted that these fragments are characteristic of ions but also of molecular ion losses.
| Masa de ion | Asignación |
| 29 | Ethyl (C2H5), formyl (CHO) |
| 30 | Nitroso (NO) |
| 31 | Methoxyl (CH3O), hydroxymethyl (CH2OH) |
| 39 | Cyclopropenyl (C3H3) |
| 41 | Allyl (CH2CH=CH2) |
| 43 | Propyl (C3H7), acetyl (CH3CO) |
| 45 | Carboxyl (COOH) |
| 46 | Nitro (NO2) |
| 55 | Butenyl (C4H7) |
| 56 | C4H8 |
| 57 | t-Butyl (C4H9), Propanoyl (CH3CH2CO) |
| 60 | Acetic acid |
| 65 | Cyclopentadienyl (C5H5) |
| 77 | Phenyl (C6H5) |
| 91 | Benzyl (tropylium, Ph-CH2) |
| 92 | Methylenepyridine (azatropylium, C5H5N-CH2) |
| 105 | Benzoyl (Ph-CO) |
| 127 | Iodine |
Fragmentation rules for organic compounds
In order to systematize as far as possible all the above indications on the fragmentation of the molecular ion, we will consider that, regardless of the type of breakage (homo or heteronuclear), the fragmentations respond to one of the following four rules:
1st Fragmentation rule
| 1st Rule! «Carbon-carbon bonds are preferentially cleaved at branch points..» |

The positive charge will remain on the most stable carbocation, the stability of these being:
Tertiary (3rd) > Secondary (2nd) > Primary (1st) > Methyl.
2nd Fragmentation rule
| 2nd Rule! «Double bonds or double bond systems (including aromatics) favor the cleavage of aryl and benzyl bonds..» |

The positive charge will normally remain, forming an aryl or benzyl carbocation. In the latter case, it should be noted that it is not a benzyl cation that is formed, but a benzyl cation that undergoes regrouping.
Consequently, it gives rise to the formation of the tropylium ion (C7H7+), which is more stable than the former as it is aromatic.

A special case is the case of cycloalkenes, which have two bonds in the allylic position. Consequently, they undergo simultaneous fragmentation of both bonds. This fragmentation is known as the retro Diels-Alder reaction.

3rd Fragmentation rule
| 3rd Rule! «The heteroatoms, as electron donors, favor the fragmentation of the bonds of the carbon atom that supports the heteroatom..» |
There are two cases to consider.
- The heteroatom is attached to the carbon by a single bond. As a consequence, either the C-X bond or the C-C-X bond could be broken, with the charge remaining on the fragment that stabilises it best. If the C-X bond is broken, the charge remains preferentially on the carbon atom.
fig-39 - The heteroatom is joined by a double bond. For example, if it is a carbonyl group (C=O), the most stable ion is usually the acyl ion (RCO+).
fig-40
4th fragmentation rule
| 4th Rule! «Double bonds and heteroatoms favor, as hydrogen acceptors, the rearrangement of a hydrogen through a cyclic six-membered transition state..» |
It is known as specific hydrogen rearrangement or McLafferty rearrangement”.

These transposons have the following main characteristics:
- It is usually evidenced by the formation of even mass ions from even molecular ions.
- For them to occur, there must be a hydrogen atom in the γ position with respect to the hydrogen acceptor double bond.
fig42