What is Bradsher cyclization?
The Bradsher cyclization, also known as the Bradsher reaction, Bradsher-type aromatic cyclodehydration, and Parham cyclization, was first reported by Bradsher in 1939. This acid-catalyzed cyclization reaction is commonly used for synthesizing various polycyclic aromatic compounds, particularly those in the phenanthrene series.
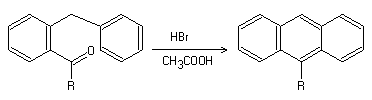
In addition, various functional groups attached to an aromatic ring have been shown to be suitable for acid-catalyzed cyclization, including ketones, aldehydes, aldehyde derivatives (such as acetals), nitriles, amino alcohols, carbinols, Grignard-reagent-converted nitriles, haloethers, olefins, olefin oxides, and glycols. Furthermore, this cyclization can also be induced by photoirradiation or a strong base.
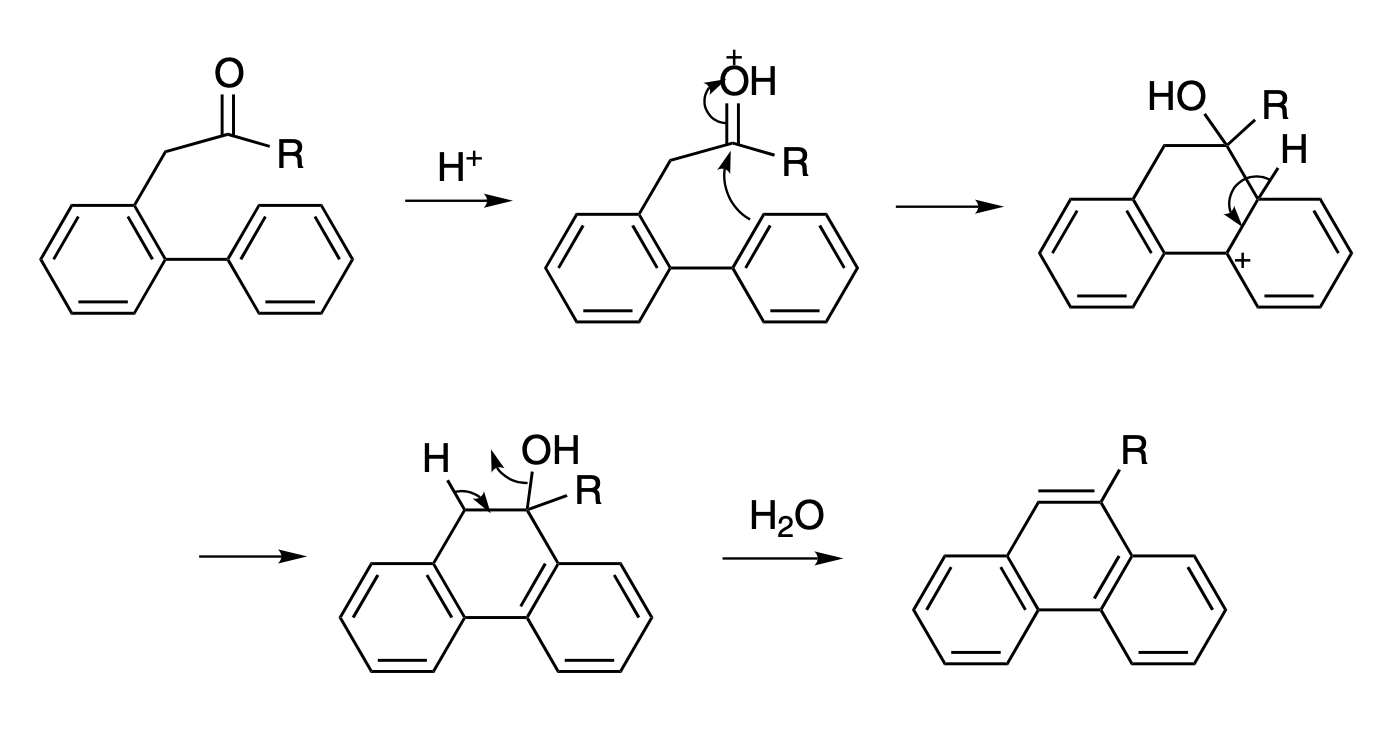
Mechanism of reaction
References
Charles K. Bradsher “Synthesis of Phenanthrene Derivatives. IV.1 9,10-Cyclopenteno- and 9,10-Cyclohexenophenanthrene”
Journal of the American Chemical Society 1939 61 (11), 3131-3132
DOI: 10.1021/ja01266a044
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.