Objective
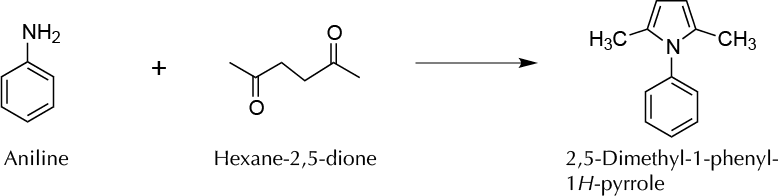
To synthesize at microscale a heterocyclic compound, in particular, 2,5-dimethyl-1-phenylpyrrole using the Paal-Knorr synthesis.
Background
Among different types of cyclization reactions for preparing nitrogen heterocycles, one of the most popular methods for the synthesis of substituted pyrroles is known as the Paal-Knorr synthesis. In this experiment, a 1,4-dicarbonyl compound is heated with a primary amine to produce a pyrrole derivative.
Microscale experimental procedure
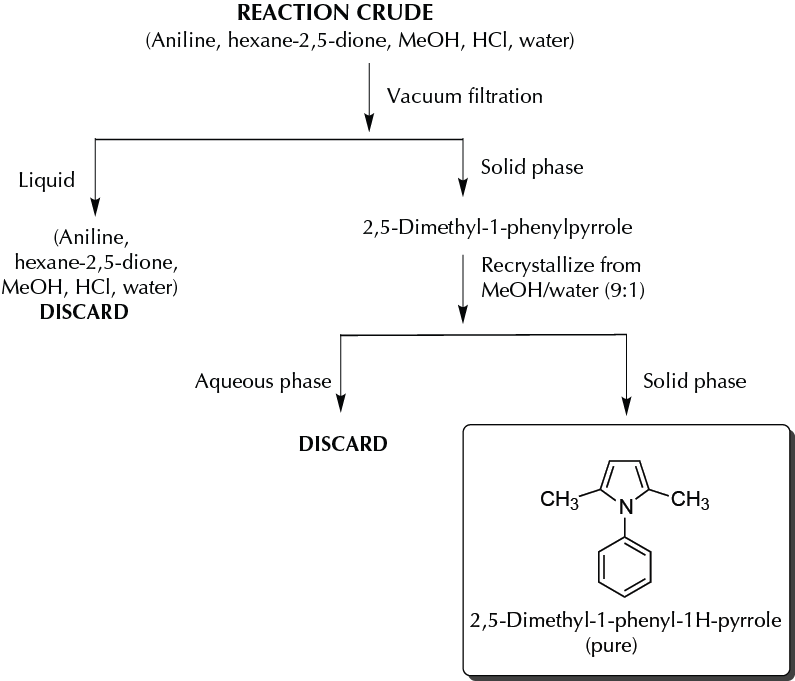
In a round-bottom flask fitted with a reflux condenser, add 186 mg (2.0 mmol) of aniline, 228 mg (2 mmol) of hexane-2,5-dione, 0.5 ml of MeOH, and 1 drop of HCl (conc.). Heat the mixture at reflux for 15 min, and then add 5.0 ml of HCl 0.5 M (keep the mixture cool in an ice bath). Collect the crystals by means of vacuum filtration, and recrystallize from 1 ml of a mixture of MeOH/water (9:1). The yield is approximately 52% (178 mg).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Aniline | 93.13 | -6 | 184 | 1.022 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Hexane-2,5-dione | 114.14 | 6 | 191 | - |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
| 2,5-Dimethyl-1-phenyl-1H-pyrrole | 171.24 | 48-52 | 156-160 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Aniline |     |
| HCl |   |
| Hexane-2,5-dione |   |
| MeOH |    |
| 2,5-Dimethyl-1-phenyl-1H-pyrrole | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Aniline | PAYRUJLWNCNPSJ-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Hexane-2,5-dione | OJVAMHKKJGICOG-UHFFFAOYSA-N |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
| 2,5-Dimethyl-1-phenyl-1H-pyrrole | JNXIFVSGXLGULI-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- R. Al-Awar and G. H. Jr. Wahl, Microscale syntheses of heterocyclic compounds, Journal
of Chemical Education 67 (1990), no. 3, 265–266, DOI: 10.1021/ed067p265
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.