Objective
To apply microscale procedures to the synthesis of three vanillin derivatives by means of functional group transformations.
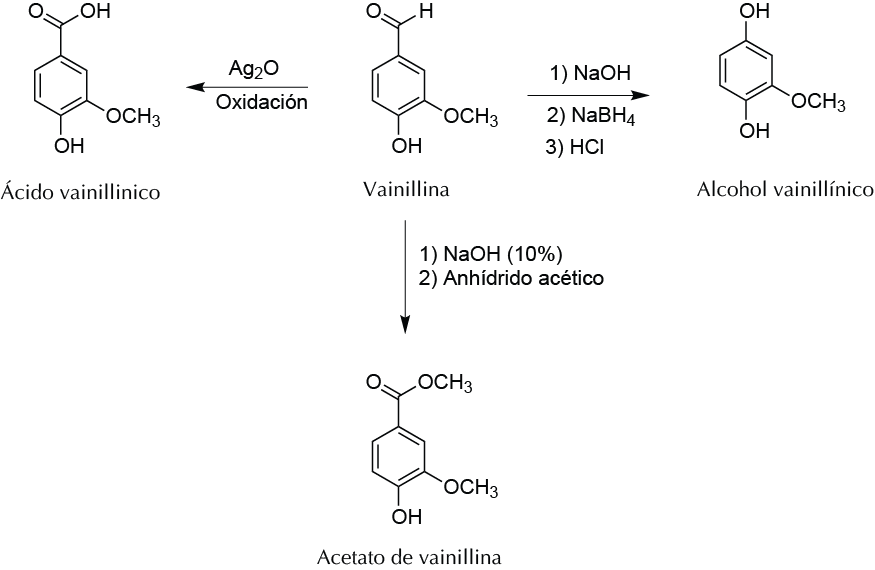
Background
Vanillin (3-methoxy-4-hydroxybenzaldehyde) is a white crystalline compound that can be isolated from vanilla plant (Vanilla planifola), the highest concentration of this compound being in the seeds. It is one of most commonly used flavorings and perfuming agents worldwide in the food and cosmetic industries, and because of its high volume of consumption, today most vanillin is produced by chemical synthesis. Vanillin is a polyfunctional compound with an aromatic ring bonded to an aldehyde group, -OH phenolic, and a methoxy group. In this experiment, three derivatives of vanillin will be prepared by relatively simple chemical reactions, leading to the formation of molecules with different aromas of the starting molecule.
Microscale experimental procedure
A) Vanillyl alcohol synthesis by reduction of the aldehyde group
Dissolve 380 mg (2.5 mmol) of vanillin in a 5 ml conical vial provided with a spin vane in 2.5 ml of NaOH 1 M solution until a yellow solution is formed. Cool the mixture in an ice-water bath at 10-15 ºC. Then add slowly 75 mg of NaBH4. After addition, continue stirring for 30 min at r.t. Then return the vial to an ice-water bath and add HCl 2.5 M dropwise until slightly acid pH (check with pH test paper). Under these conditions, a precipitate that is the final reaction product should appear. If crystallization does not occur, scrape the flask wall with a microspatula gently until the first solid crystals appear, and after complete crystallization, let stand at r.t. The estimated yield is approximately 65 %. Isolate the solid by vacuum filtration in a Hirsch funnel and purify it by recrystallization from ethyl acetate (m.p. = 108-109 ºC).
B) Synthesis of vanillic acid by oxidation of the aldehyde group
In a conical vial with a spin vane, dissolve 170 mg AgNO3 in 1 ml of deionized water. Add dropwise 0.5 ml of NaOH 2.5 M solution, stir the mixture until the precipitation of Ag2O, and continue stirring for an additional 5 min. After this, stop stirring to let the solid deposit in the bottom of the vial. Then remove the supernatant liquid with a Pasteur pipette, and wash the remaining solid by adding 4 × 1 ml of deionized water. Stir and discard the Ag2O, allowing it to settle to the bottom each time to eliminate the nitrates completely. After this step, add to the solid 2.5 ml of a solution of NaOH 2.5 M, and heat the vial with a water bath to 60 ºC. When this temperature is reached, slowly add 152 mg (1 mmol) of vanillin using a microspatula for approximately 15 min. A metallic silver depot will form on the vial walls (silver mirror), which will subsequently fall to the bottom of the vial and form a yellow solution. Transfer the yellow solution to an Erlenmeyer and wash the remaining solid in the vial with 3 × 1 ml of deionized water. Wash with water and collect the yellow solution, and to this add HCl 6 M dropwise until reaching an acidic pH (check with pH test paper). Put the flask into an ice-water bath to trigger the precipitation of the final product. If this does not occur, gently scrape the wall of the vial with a microspatula until crystallization begins. Vacuum filter the solid in a Hirsch funnel, and, when dried, weigh and calculate the yield (estimated yield 60 %). The product may be recrystallized from water (1.2 ml of water per 100 mg product).
C) Preparation of vanillin acetate (esterifying the phenolic -OH)
Dissolve 304 mg of vanillin (2 mmol) in a 50 ml Erlenmeyer containing 5 ml of NaOH 10 % with magnetic stirring. Add 6 g of crushed ice and 1.6 ml of acetic anhydride, cover the flask with a septum, and shake the mixture at r.t. for 15 min. Vacuum filter the solid with a Hirsch funnel. Dry, weigh, and calculate the yield (estimated yield 35 %). Purify the product by recrystallization from EtOH/water (1:4).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| Ag2O | 231.74 | - | - | 7.143 |
| AgNO3 | 169.87 | 212 | 440 | 4.350 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaBH4 | 37.8 | 400 | - | 1.07 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Vanillic acid | 168.15 | 208-211 | - | - |
| Vanillin | 152.15 | 81-83 | 170 | 1.056 |
| Vanillin acetate | 194.18 | 77-79 | - | - |
| Vanillyl alcohol | 154.16 | 110-117 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic anhydride |    |
| Ag2O |   |
| AgNO3 |    |
| EtOH |  |
| Ethyl acetate |   |
| HCl |   |
| NaBH4 |    |
| NaOH |  |
| Vanillic acid | Non-hazardous |
| Vanillin |  |
| Vanillin acetate | Non-hazardous |
| Vanillyl alcohol |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| Ag2O | KHJDQHIZCZTCAE-UHFFFAOYSA-N |
| AgNO3 | SQGYOTSLMSWVJD-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaBH4 | YOQDYZUWIQVZSF-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Vanillic acid | WKOLLVMJNQIZCI-UHFFFAOYSA-N |
| Vanillin | MWOOGOJBHIARFG-UHFFFAOYSA-N |
| Vanillin acetate | PZSJOBKRSVRODF-UHFFFAOYSA-N |
| Vanillyl alcohol | ZENOXNGFMSCLLL-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- R. G. Fowler, Microscale reactions of vanillin, Journal of Chemical Education 69 (1992),
no. 2, A43-A44, A46, DOI 10.1021/ed069pA43