What is the Diels-Alder reaction mechanism?
The Diels-Alder (DA) reaction is the pericyclic reaction par excellence, also called [4+2] cycloaddition.
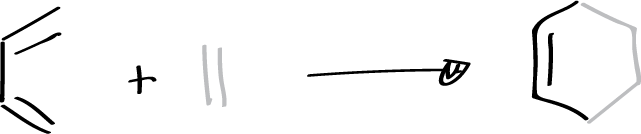
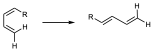
It is a reaction in which a conjugated diene combines with a so-called dienophile, which can be an alkene or alkyne, to give a 6-membered cyclic adduct. The simplest case is the reaction between buta-1,3-diene and ethene. The diene contributes 4 π electrons and the dienophile 2 (π4+π2) as shown in the figure.
The diene and dienophile react on the same side of both molecular planes (suprafacially) as shown in the figure below:
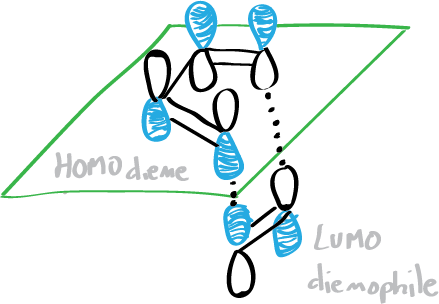
Let’s take a look at its reaction mechanism and try to explain why the most significant features of this reaction occur.
Main characteristics of the Diels-Alder reaction
This reaction has the following experimental characteristics:
The reaction with the unsubstituted reagents, i.e., butadiene and ethylene, takes place at moderate temperatures, the reagents must be heated and placed at a certain pressure, but the reaction takes place with activation energies that are compatible with the state correlation diagram.
- It is observed that if we substitute both the diene and the dienophile the reaction proceeds more easily especially if these substituents have complementary electronic characteristics, i.e., if we put an electron donor substituent in one of the reactants in the other it must be electron attracting. Thus we know DA reactions in which there are electron-donating substituents in the diene and electron-attracting substituents in the dienophile, which are the most normal and occur very easily, but the opposite is also known. From a mechanistic point of view there is no difference between them.
- The diene will be in equilibrium between the s-cis and s-trans conformation, the s-trans being the most stable. Therefore, for the reaction to occur in acyclic dienes, they must adopt by rotation of the single bond a conformation called s-cis.

Anything that favors the s-cis conformation will also favor the DA reaction. To the extent that if the diene cannot adopt that conformation, the reaction will not occur.

On the contrary, if the diene is forced to the s-cis conformation, the reaction will take place very easily.

Cyclopentadiene is a structure in which the s-cis conformation is already forced and therefore gives reactions very easily and is therefore one of the most commonly used dienes in DA reactions.
- It is a pericyclic reaction, since the formation of the adduct occurs in a single step via a cyclic transition state, in this case, of six π electrons.
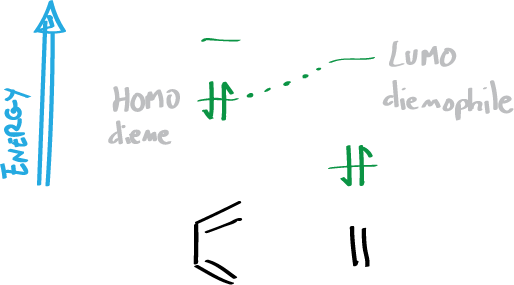
- The reaction takes place by the overlapping of the molecular orbitals (MO) closest in energy. That is, the higher energy occupied MO (HOMO) of the diene and the lower energy empty MO (LUMO) of the dienophile.
In suitably substituted dienes and dienophiles, the reagent approach allows two types of adducts: endo and exo, with the endo-type attack being preferred.
- The reaction is stereospecific with respect to the substituents on both the diene and dienophile, since the relative cis- or trans- stereochemistry of the reactants is maintained in the adduct.
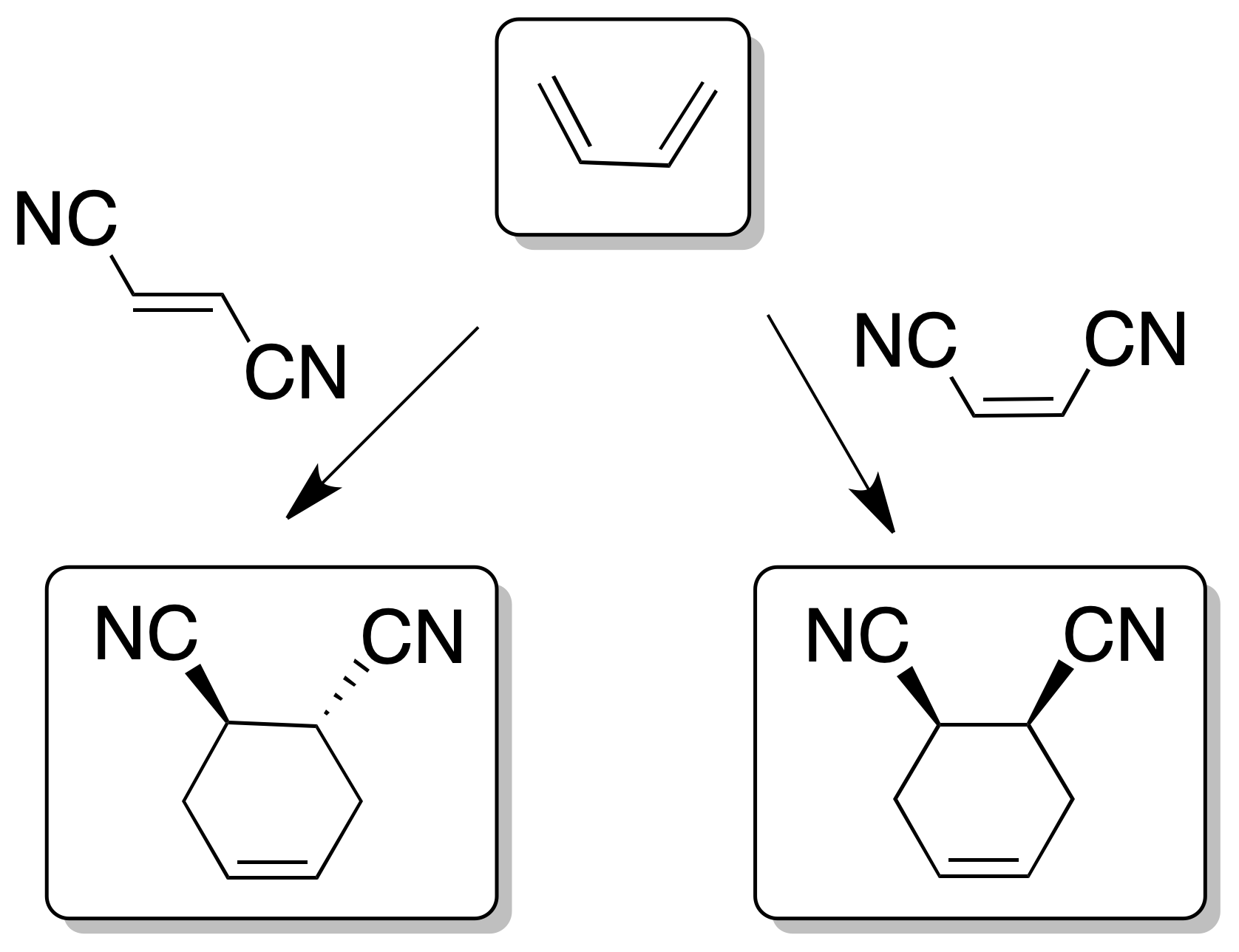
- The reaction is favored when there are electron-donor groups on the diene such as -R, -OR, -OSi(R)3, -NH2, etc., (increase the energy of the HOMO) and electron-acceptor on the dienophile such as -CHO, -COR, -COOR, -CN, >C=C<, -NO2, -Ph or -X (decrease the energy of the LUMO). As a result, the two orbitals HOMOdiene and LUMOdienophile become closer in energy favoring the reaction.
- Steric hindrance on the atoms directly involved in the reaction slows it down.
- The Diels-Alder reaction may be intramolecular.
- The Lewis acids catalyze the reaction.
How do the substituents affect the reaction?
Bulky substituents at position 1 of the diene favor the s-trans conformation and will therefore slow down the reaction.
On the contrary, if we have a bulky substituent in position 2, a stronger interaction can occur in the s-trans conformation than in the s-cis conformation, and therefore bulky substituents in position 2 accelerate the reaction.

- In general, medium activation energies are observed which are compatible with the small barrier observed in the correlation diagrams and, above all, quite large activation entropies are observed which are compatible with a very ordered ET, which suggests that the ET must be very ordered by means of a pericyclic type mechanism.
- When both the diene and the dienophile are substituted a characteristic regiochemistry is observed depending on the position of the substituent. If the diene and the dienophile are substituted and the diene is substituted at position 1 regardless of the electronic characteristics of the substituents there will be two possible regiochemistries so that the one that places the two substituents as close as possible will be obtained as the major product:
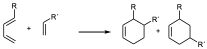
major
When the substituent in the diene is in position 2, two different regiochemicals can also occur, but in this case the major product is the one that places the two groups as far apart as possible.
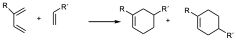
major
For this reason, the DA reaction is referred to as the ortho and para orientation versus the target. Associating the arrangements 1,2 in aromatic systems to ortho and 1,4 to para, and 1,3 to meta. That is to say that whenever there may be the possibility of substituted reactants in both diene and dienophile it will preferably be obtained when the two groups are as close as possible or as far apart as possible.
Main reaction characteristics: HOMO-LUMO interactions
We will try to explain some of these characteristics taking into account that these are pericyclic reactions.
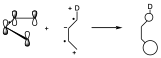
We will first try to explain the fact that the reaction occurs more easily as the substituents have specific electronic characteristics. If the diene and dienophile have substituents with complementary electronic characteristics the reaction will proceed more easily. One of the possible explanations for the reaction is that the HOMO of one component interacts with the LUMO of the other, and that both the HOMO and LUMO have the appropriate symmetry, and that this interaction is as favorable as possible. Let us look at the orbitals of the reactants in the unsubstituted ethylene and butadiene. With respect to the symmetry element which is the bisecting plane all the orbitals have that symmetry.

The interaction of the HOMO of the diene with the LUMO of the dienophile can take place giving rise to two new orbitals in such a way that the two electrons are located in the lowest energy one, and on the other hand it can give rise to the interaction of the HOMO of the dienophile with the LUMO of the diene that still have the same symmetry. We see that there are two interactions, both of equal intensity, but taking into account that the interaction is taking place between levels of very different energy.
Let us suppose now that of the two possibilities that we have said, that of the diene with an electron donor substituent and the dienophile with an electron attractor. If this is so, if we are giving more electrons to a dienic system, what we do is to put in excess the number of electrons of the system and therefore we are raising the energy of each of those levels, therefore the energy levels of the π system of the diene will be elevated in energy with respect to the previous ones, both the occupied levels and the virtual ones. On the other hand, if we add an electron-attracting substituent to the dienophile, it will be a π system from which electrons are being withdrawn, and therefore we are stabilizing the π system, we are lowering its energy. The symmetry remains the same.
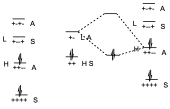
The result of this process is that the HOMO of the dienophile and the LUMO of the diene are very far apart in energy, but on the contrary the HOMO of the diene and the LUMO of the dienophile are very close so that before two small interactions could occur because the energy differences were very large. But here as the energy difference is small only one interaction will occur but very stabilizing and therefore the reaction occurs with a lower activation energy and therefore occurs more easily.
We could have done the same by changing the electron donor and electron acceptor substituents in the diene and dienophile, so that we would have an interaction between the HOMO of the dienophile and the LUMO of the diene.
The fact is that the two possible interactions produce only one, but that one is much more stabilizing than the two that were produced before.
Let’s consider that we have the diene with an electron donor substituent at position 1 and the electron attracting dienophile.
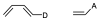
Let’s see why the regiochemistry we have seen is favored. There is going to be the interaction between the HOMO of the diene and the LUMO of the dienophile. Let’s look at the qualitative shape of those molecular orbitals to see how it can produce the interaction. The HOMO of the diene is an orbital where there is a node in the center.

If we have a substituent attached in position 1 and which is an electron donor, a dienic system with an excess charge can be associated with the pentadienyl anion, i.e., the pentadienyl anion would be the extreme if we consider that the electron donor substituent is a net negative charge.

If we have the pentadienyl anion system, the HOMO will be the third one since we will have three levels occupied. The HOMO places us the ocntributions of each atom in this way.
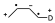
If we combine this with the above, we will obtain a small contribution in the first atom and a large one in the fourth atom independently of what happens in atoms 2 and 3 since they will be the extremes interacting with the dienophile.
On the other hand, the 2-electron system is a double bond with an electron-attracting substituent, so that it is the LUMO of this system that interacts with the HOMO of the diene. The system that we can equate to a double bond with an electron-attracting substituent is the allyl cation at its maximal end. The allyl cation will have the following form:
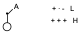
Therefore, in the LUMO orbital there will be a null contribution in one atom and a large contribution in another.
Therefore, regardless of the signs, which can be changed, the qualitative forms of the HOMO of the diene and the LUMO of the dienophile are these two.
The two possible interactions that can occur are those that cause the small lobe to interact with the small lobe and the large lobe to interact with the large lobe, or if we get the other regiochemistry, it would be the large lobe with the small lobe and the small lobe with the large lobe.

The interaction that will preferably occur will be the one in which the maximum overlap occurs, which will be the first one. The effective overlap of that regiochemistry is better than the second one.
What would it look like when we have an electron donor substituent on the diene at position 2, and an electron attracting substituent on the dienophile?
It is necessary to consider the mixing of the diene HOMO (which is the one that is going to act) with the allyl anion system.
Resumen de interacción HOMO-LUMO
Through the study and analysis of the HOMOdiene and LUMOdienophile molecular orbitals, it is possible to predict the orientation of the attack between the reactants and, therefore, the position of the substituents in the adduct that is obtained in higher proportion.
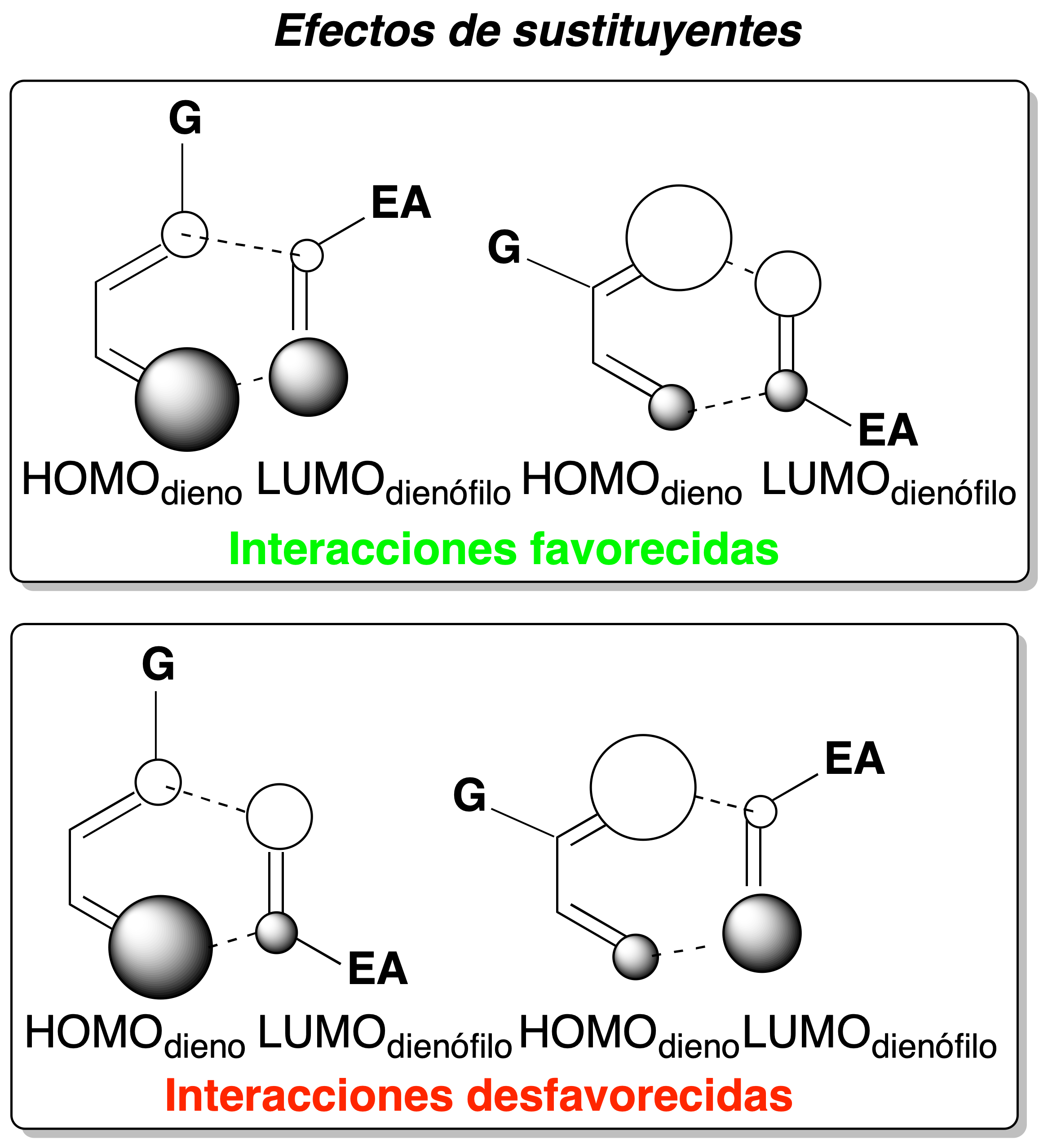
The following considerations may be taken into account for this purpose:
- Substituents on dienes and dienophiles modify the size of the HOMO and LUMO orbitals.
- The vast majority of substituents in position 1 of buta-1,3-diene increase the size of the HOMOdiene at 4 position.
- Most of the substituents in the 2-position of buta-1,3-diene increase the size of the HOMOdiene at the 1-position.
- The electron-attracting groups on the dienophile increase the size of the LUMOdienophile at 2 position.
- The orientation of the attack is the one that favors the maximum overlapping of orbitals (interaction with large-large and small-small orbitals).
References
- Diels, O. and Alder, K. (1928), Synthesen in der hydroaromatischen Reihe. [Syntheses in the hydroaromatic series.] Justus Liebigs Ann. Chem., 460: 98-122. https://doi.org/10.1002/jlac.19284600106
- Diels, O. and Alder, K. (1929), Synthesen in der hydroaromatischen Reihe. III. Mitteilung: Synthese von Terpenen, Camphern, hydroaromatischen und heterocyclischen Systemen. Mitbearbeitet von den Herren Wolfgang Lübbert, Erich Naujoks, Franz Querberitz, Karl Röhl, Harro Segeberg. [Syntheses in the hydroaromatic series. III. communication: synthesis of terpenes, camphors, hydroaromatic and heterocyclic systems. Co-edited by Wolfgang Lübbert, Erich Naujoks, Franz Querberitz, Karl Röhl, Harro Segeberg.] Justus Liebigs Ann. Chem., 470: 62-103. https://doi.org/10.1002/jlac.19294700106
- Diels, O. and Alder, K. (1929), Synthesen in der hydroaromatischen Reihe, IV. Mitteilung: Über die Anlagerung von Maleinsäure-anhydrid an arylierte Diene, Triene und Fulvene (Mitbearbeitet von Paul Pries). [Syntheses in the hydroaromatic series, IV. communication: On the addition of maleic anhydride to arylated dienes, trienes and fulvene (co-edited by Paul Pries).] Ber. Dtsch. Chem. Ges. A/B, 62: 2081-2087. https://doi.org/10.1002/cber.19290620829
- Diels, O. and Alder, K. (1929), Synthesen in der hydroaromatischen Reihe, V. Mitteilung: Über Δ4-Tetrahydro-o-phthalsäure (Stellungnahme zu der Mitteilung von E. H. Farmer und F. L. Warren: Eigenschaften konjugierter Doppelbindungen (VII). [Syntheses in the hydroaromatic series, V. Communication: On Δ4-tetrahydro-o-phthalic acid (comment on the communication by E. H. Farmer and F. L. Warren: Properties of conjugated double bonds (VII).] Ber. Dtsch. Chem. Ges. A/B, 62: 2087-2090. https://doi.org/10.1002/cber.19290620830