Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To perform the bromination of an alkene, avoiding using bromine Br2 reagent.
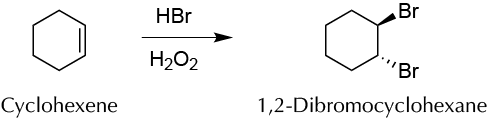
Background
The addition of halogens to alkenes is a process of great interest in Synthetic Organic Chemistry. The classical procedure is performed from the alkene and halogen. For bromination, the reagent used is bromine (Br2), which carries certain drawbacks and risks in handling. At room temperature, bromine is a reddish-brown liquid that has a strong tendency to evaporate. It is often marketed in glass ampoules, which must be carefully opened, and contents quickly added to an organic solvent, usually CCl4, in a fume hood. Its fumes are toxic and can cause skin burns.
2HBr + H2O2 ↔ Br2 + 2H2O
The procedure described in this experiment involves bromination of an alkene, generating elemental bromine from HBr solution and hydrogen peroxide H2O2. The halogen is generated in situ, making it easier to measure, transfer, and store reagents that generate bromine than bromine itself.
Experimental procedure
Add to a 100 ml round-bottom flask 2.4 ml of H2O2 30 % and a stir bar. Place the flask on a hot plate, fixing it to a support with a clamp. The flask is attached a water-jacketed condenser. There is no need to connect it to the cooling circuit.
When stirring begins, carefully add, with an eyedropper, 40 drops (approximately 1.6 ml) of HBr in acetic acid from the top of the condenser, so that the drops fall directly without touching the walls. The reaction mixture will take on a reddish color due to the formation of Br2.
| DANGER! “Work in a fume hood and wear gloves.” |
Once the addition of HBr is finished, add 40 drops (approximately 1.4 ml) of cyclohexene, again taking care that the drops fall directly into the flask without touching the walls of the condenser. When the reaction mixture changes color (becomes transparent first and then yellow), the reaction is finished.
Transfer the contents of the flask into a separatory funnel and extract with two portions of 15 ml of CH2Cl2. It is desirable for the first amount of CH2Cl2 to be used to wash the flask containing the crude reaction in order to dissolve the product that may remain inside. Combine the organic layers and wash once with a solution of NaHSO3 in the funnel. After washing, transfer to a 100 ml Erlenmeyer. Dry the solution with anhydrous sodium sulfate, remove the desiccant by gravity filtration into a tared round-bottom flask, and remove the solvent under reduced pressure while keeping the bath of the rotary evaporator at 40 ºC, as the reaction product has a b.p. of 145 ºC. Weigh the flask and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Cyclohexene | 82.14 | -104 | 83 | 0.779 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| H2O2 | 34.01 | - | - | 1.110 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| NaHSO3 | 104.06 | - | - | 1.48 |
| trans-1,2-Dibromocyclohexane | 241.95 | - | 145 | 1.784 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Cyclohexene |    |
| CH2Cl2 |  |
| H2O2 |   |
| Na2SO4 | Non-hazardous |
| NaHSO3 |   |
| trans-1,2-Dibromocyclohexane | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Cyclohexene | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| H2O2 | MHAJPDPJQMAIIY-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| NaHSO3 | DWAQJAXMDSEUJJ-UHFFFAOYSA-M |
| trans-1,2-Dibromocyclohexane | CZNHKZKWKJNOTE-PHDIDXHHSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- L. C. McKenzie, L. M. Huffman, and J. E. Hutchison, The evolution of a green chemistry laboratory experiment: greener brominations of stilbene, Journal of Chemical Education 82 (2005), no. 2, 306, DOI 10.1021/ed082p306