Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To conduct the Friedel-Crafts reaction under low environmental impact with a reusable catalyst.
Background
Alkylation of aromatic compounds is a remarkable reaction both at laboratory and at industrial scale. Such reactions are catalyzed by Lewis acids. One of the most widely used for its effectiveness is AlCl3. When the reaction occurs between an alkyl halide and an aromatic derivative, Lewis acid weakens the carbon-halogen (C–X) bond, forming an electrophilic carbocation that reacts with the aromatic ring. Often, transpositions occur in the carbocation formation, leading to mixtures of replacement product. Moreover, once the reaction ends, treatment with an aqueous medium to remove AlCl3 from the reaction crude is required.
In the search for new synthetic procedures respectful of environment, graphite has been found to be capable of catalyzing the alkylation reaction of aromatic compounds with secondary, tertiary, and benzyl halides, while primary halides do not react. The reaction mechanism is not fully understood.
This procedure is within the principles advocated by Green Chemistry, because, under these conditions, the formation of byproducts and waste is minimized, the toxicity of the compounds being handled is reduced, and especially catalyst can be re-used.
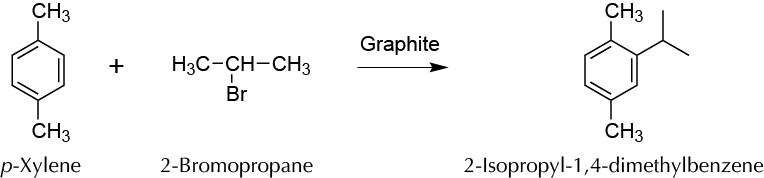
Experimental procedure
Add 5 ml of p-xylene, 0.44 ml of 2-bromopropane, and 0.5 g of graphite to a 100 ml round-bottom flask equipped with a stir bar and then attach a water condenser and reflux for 90 min. At the end of the reaction time, cool the flask to room temperature.
Vacuum filter the reaction crude with a Hirsch funnel or a Büchner. Wash the graphite with 15 ml of hexane, which joins to the filtrate. Transfer the result of filtration and wash to an round-bottom flask, and evaporate under reduced pressure (rotary evaporator) to remove the solvents. Weigh the reaction product, which is a liquid, to calculate the yield. Once the catalyst is dried, it is ready for use again in another reaction.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Graphite | 12.01 | 3,800 | - | 1.900 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| p-Xylene | 106.17 | 13.0 | 138.4 | 0.860 |
| 2-Bromopropane | 122.99 | -89 | 59 | 1.310 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Graphite |  |
| Hexane |     |
| p-Xylene |   |
| 2-Bromopropane |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Graphite | |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| p-Xylene | URLKBWYHVLBVBO-UHFFFAOYSA-N |
| 2-Bromopropane | NAMYKGVDVNBCFQ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- G. A. Sereda and V. B. Rajpara, A green alternative to aluminum chloride alkylation of xylene, Journal of Chemical Education 84 (2007), no. 4, 692, DOI: 10.1021/ed084p692