Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To produce n-butyl bromide from n-butyl alcohol, and to demonstrate the ability to carry out a substitution reaction in organic chemistry, specifically the conversion of an alcohol to an alkyl halide.
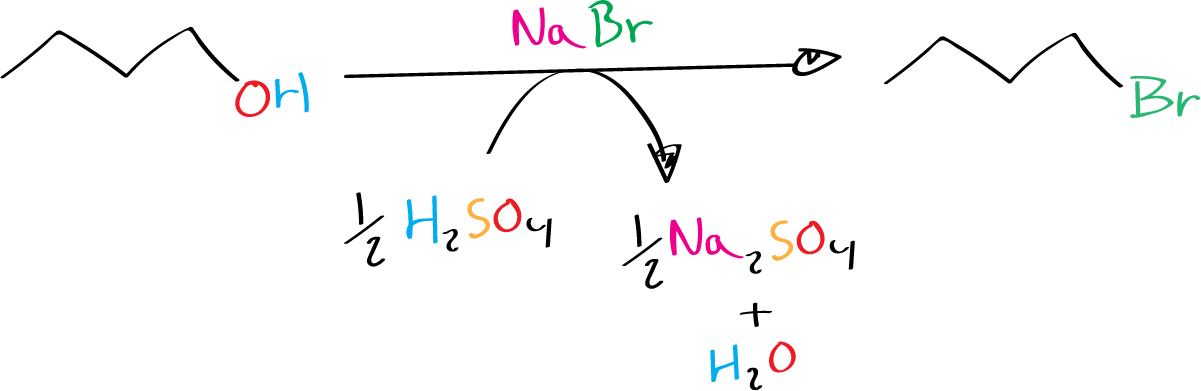
Background
Reacting a primary alcohol with a hydrogen halide produces a primary alkyl halide. The reaction follows an SN2 mechanism, as the dehydration step is competitive. The reaction requires a strong acid to protonate the hydroxyl group. Aqueous HBr, HI or HBr gas can be used. In this experiment, the HBr is generated in situ by reacting NaBr with H2SO4.
NaBr + H2SO4 → HBr + NaHSO4
In this transformation, some side reactions occur: The butan-1-ol can react with the HSO– ions present in the solution to yield a hydrogen sulfate ester (R−OSO3H). This inorganic ester can in turn trigger an elimination reaction that leads to but-1-en (a gas reflux is lost during processing or during the reaction) or replacement with butan-1-ol to yield di-n-butyl ether (which must be removed during the processing of the reaction).
Experimental procedure
In a 250 ml round-bottom flask, place 30 g of sodium bromide and 30 ml of water. Shake the flask until most of the salt has been dissolved. Add 18.5 g of butan-1-ol, and cool the flask to 5–10 ºC in an ice bath. Slowly add 25 ml of concentrated H2SO4.
Place the flask with a reflux condenser and a trap adapted for gases containing NaOH solution (aqueous 5% NaOH), and reflux the mixture for 30 min with magnetic stirring.
| DANGER! “During the refluxing, HBr vapors are are released, which are corrosive and toxic (10–20 times more toxic than CO2 and as toxic as Cl2). The reaction should be performed in a fume hood and the reflux system connected to a trap for HBr. Make sure that in the trap, the funnel is not submerged, since liquid may enter in the reaction flask.” |
During the reflux the reaction mixture forms two layers. Cool the reaction flask (ice bath can be used) to a temperature at which it can be manipulated. Add a magnetic bar and prepare the assembly for a simple distillation. Distill until the temperature of the distillation mixture reaches 110–115 ºC. The distillate consists of two phases (1-bromobutane and water), which are most apparent at the beginning of the distillation. At the end of the distillation, 1-bromobutane should no longer be visible in the drops of distillate. “To check that there is no codistillation of oil with the water at the end of the distillation, collect a few milliliters of distillate in a test tube. Shake the test tube, and if there is oil, droplets will be visible. The distillation residue (strong acid!) must be collected by pouring onto ice and diluting with water (pour to the acid waste container).
Transfer the distillate to a 125 ml separatory funnel, and add approximately 25 ml of water. Shake the funnel and allow the two phases to separate. Collect the lower layer of the 1-bromobutane in an Erlenmeyer flask. “Be very careful and pay attention to the identification of the phases in the funnel during this experiment. It is wise to keep all layers in labeled flasks until the end of the experiment to avoid accidentally pulling the wrong layer. Discard the top layer. Add 25 ml of concentrated H2SO4 and cool the 1-bromobutane with ice.
Swirl the flask to mix the contents. If the mixture starts to heat, cool the flask in an ice bath. Pass the mixture to a separatory funnel. The concentrated H2SO4 is denser (d = 1.84) than 1-bromobutane (d = 1.28), and therefore 1- bromobutane now forms the upper layer. Gently shake the separatory funnel to avoid the formation of an emulsion and let stand for 5–10 min. The formation of emulsions is frequent in extration and will require a rest period for the two phases to separate.
| DANGER! “Extreme care is needed when handling concentrated H2SO4 in the funnel. The funnel should be frequently turned on to release the excess pressure. An oversight, even a leaky stopcock, can lead to the H2SO4, spilled on clothing or the work area. Any spillage onto skin or clothing should be washed immediately with water.” |
Collect lower layer (caution: strong acid) and discard; pour over ice and dilute with water; eliminate in the acid waste container. Wash the 1-bromobutane remaining in the funnel with 25 ml of water to remove the residual H2SO4. As 1-bromobutane is denser than the aqueous solution, 1-bromobutane will now form the lower layer. Shake the funnel, collecting this lower layer into a clean flask. Remove the aqueous phase remaining in the funnel and replace 1-bromobutane in the funnel. Extract the 1-bromobutane with 25 ml of a solution of 10% NaOH. In this extraction, as in the above, 1-bromobutane is in the lower layer in the separatory funnel.
Collect 1-bromobutane in another clean flask, add 2 g of CaCl2 anhydrous, stopper the flask tightly and let the mixture stand to clarify the liquid (best overnight). Since the 1-bromobutane is quite volatile, the drying vessel should have a glass stopper or septum.
Decant the clean 1-bromobutane to a 50 ml round-bottom flask using a disposable dropper to transfer remaining liquid. Be very careful not to transfer any solid calcium chloride CaCl2. Add a magnetic bar and distill 1-bromobutane. Collect the fraction at 98–103 ºC (if the distillate is cloudy it means it has moisture and should be dried and redistilled). Estimated yield will be 50 %.
If the experiment cannot be completed in one session, the breakpoint is after the reflux period: while the 1-bromobutane is being dried with anhydrous CaCl2.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1-Bromobutane | 137.02 | -112 | 100-104 | 1.276 |
| Butan-1-ol | 74.12 | -90 | 116-118 | 0.810 |
| CaCl2 | 110.98 | 782 | >1,600 | 2.15 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1-Bromobutane |    |
| Butan-1-ol |    |
| CaCl2 |  |
| H2SO4 |  |
| NaOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1-Bromobutane | MPPPKRYCTPRNTB-UHFFFAOYSA-N |
| Butan-1-ol | LRHPLDYGYMQRHN-UHFFFAOYSA-N |
| CaCl2 | UXVMQQNJUSDDNG-UHFFFAOYSA-L |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145