Objective
To perform a non-conventional hydrogenation of a vegetable oil (olive, palm, sunflower seed, etc.) to produce margarine.
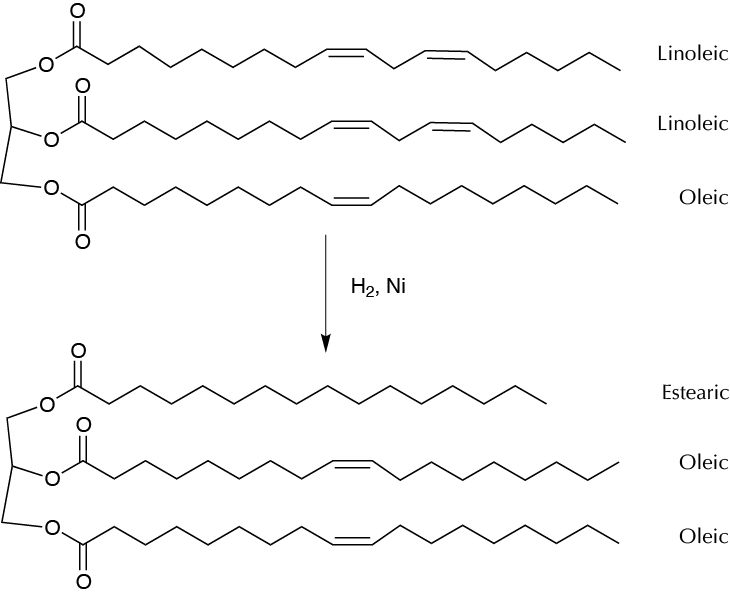
Background
Margarine has traditionally been manufactured by hydrogenation of vegetable oils (olive, palm, sunflower seed, etc.) by an industrial process for converting these oils into a solid, stable, and spreadable substance.
The procedure followed in this experiment is the partial hydrogenation of a sample of olive oil using cyclohexene as the hydrogen source in the presence of Pd on carbon, which is used as catalyst. Under these conditions, cyclohexene is a hydrogen source that is converted in situ into benzene, which is added to the double bonds of the fatty acids comprising part of oil molecules. The reaction is the so-called heterogeneous catalytic transfer hydrogenation.
Microscale experimental procedure
In a conical 5 ml vial with a spin vane, add 600 mg of olive oil, 12 mmol of cyclohexene, and 40-50 mg of Pd (on C 10%). Reflux the mixture with stirring for 50 min.
Use as a filter a Pasteur pipette attached to a support by a clamp, with a small cotton ball in the narrowest part. Fill it with 2 or 3 cm of Celite. Moisten the Celite with 2 or 3 ml of solvent (diethyl ether or CH2Cl2). Filter the catalyst in the above-described device, forcing with compressed air filtration if necessary, passing 1 ml of additional solvent to a previously tared round-bottom flask, to collect the liquid that comes out the end of the pipette.
The catalyst should be retained in the Celite. Collect the filtrate in the round-bottom flask, and then remove the solvent under reduced pressure (rotary evaporator). Determine the mass of the product in order to calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Celite® S | - | - | - | - |
| Cyclohexene | 82.14 | -104 | 83 | 0.779 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| Pd(C) | 106.42 | - | - | - |
| Vegetable oil |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Celite® S |  |
| Cyclohexene |    |
| CH2Cl2 |  |
| Diethyl ether |   |
| Pd(C) | Non-hazardous |
| Vegetable oil |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Celite® S | |
| Cyclohexene | HGCIXCUEYOPUTN-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| Pd(C) | |
| Vegetable oil |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- R. A. W. Johnstone, A. H. Wilby, and I. D. Entwistle, Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds, Chemical Reviews 85 (1985), no. 2, 129–70, DOI: 10.1021/cr00066a003