Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To produce anthrone at microscale by reduction of one of the anthracene carbonyl groups.
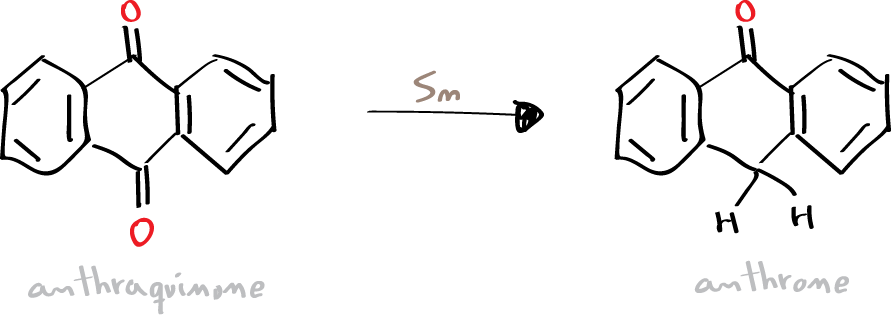
Background
Anthrone is a tricyclic aromatic ketone that can be used, for example for calorimetric determinations of carbohydrates in biological fluids. It can be synthesized by partial reduction of anthraquinone with several reagents, as sodium hydrogen sulfite NaHSO3, tin chloride SnCl2, or tin. In this experiment, the partial reduction is carried out with tin, HCl, and acetic acid as a solvent.
Microscale experimental procedure
Mix, in a conical vial with a spin vane, 104 mg (0.5 mmol) of anthraquinone, 100 mg of granulated tin, and 800 μl of acetic acid. Fit a water condenser, and reflux the mixture for 1.5 h, add dropwise 260 μl of concentrated HCl along this period to the boiling the mixture. Afterward, anthraquinone should be dissolved; if not, add more tin and HCl until complete dissolution. Remove the condenser and vacuum filter the reaction crude while hot using a Hirsch funnel to eliminate any solids in suspension. The anthrone will crystallize when the solution cools to 10 ºC (use an ice-water bath to encourage the crystallization). Vacuum filter the white solid with a Hirsch funnel and dry the solid with an air stream in the funnel. Weigh the final product, calculate the yield (estimated yield 60 %), and determine the melting point (156 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Anthraquinone | 208.21 | 284-286 | 379-381 | 1.440 |
| Anthrone | 194.23 | 154-157 | - | - |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Tin | 118.71 | 231.9 | 2,270 | 7.310 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Anthraquinone |  |
| Anthrone |  |
| HCl |   |
| Tin | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Anthraquinone | RZVHIXYEVGDQDX-UHFFFAOYSA-N |
| Anthrone | RJGDLRCDCYRQOQ-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Tin | ATJFFYVFTNAWJD-BJUDXGSMSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145
- K. H. Meyer, Anthrone, Organic Syntheses 8 (1928), 8–9, DOI: 10.15227/orgsyn.008.0008