Objective
The objective of the experiment is to dimerize the iophine product obtained from the previous step using either potassium ferricyanide or sodium hypochlorite as an oxidizing agent, in order to form a photochromic dimer.
Background
Photochromic dimers are molecules that can switch between two stable isomeric forms, known as the open and closed forms, upon excitation by light. This process involves the breaking and forming of chemical bonds within the molecule, resulting in a change in its optical and electronic properties. The open form typically has a higher energy and is less stable than the closed form, which is usually colored or fluorescent. The conversion between the two forms can be triggered by a specific wavelength of light, known as the “switching” wavelength.
The unique properties of photochromic dimer have led to their applications in a wide range of fields, including imaging, and data storage. For example, photochromic dimers have been used in the design of photoresponsive materials, such as photochromic glasses and coatings, which can switch between opaque and transparent states upon exposure to light. They have also been used in the development of molecular switches and logic gates, which can be controlled by light.
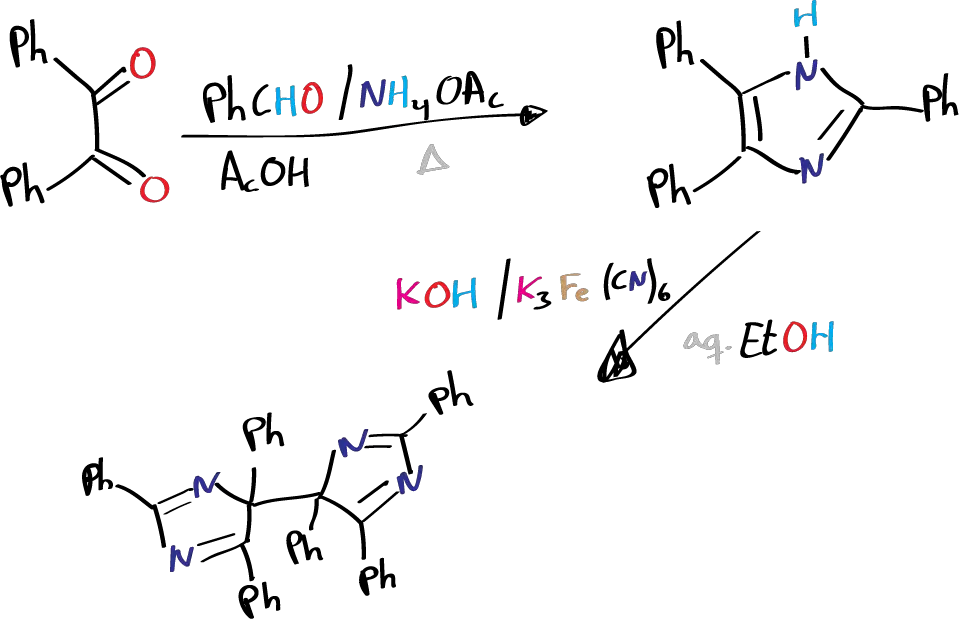
The experiment involves two main steps. Firstly, the synthesis of 2,4,5-triphenylimidazole (lophine) is carried out by condensing benzil, benzaldehyde, and ammonium acetate in acetic acid. The resulting product is purified and characterized.
In the second step, an oxidative dimerization reaction is conducted using potassium ferricyanide K3[Fe(CN)6]. Alternatively, sodium hypochlorite NaOCl can also be used as an oxidizing agent. The objective of this step is to dimerize the iophine product obtained from the previous step to form the final product. The final product is also purified and characterized to confirm its identity.
Experimental procedure
A) Preparation of 2,4,5‐triphenylimidazole
In a 250 mL round-bottom flask containing a magnetic stir bar, dissolve benzil, benzaldehyde, and ammonium ethanoate in 100 mL of ethanoic acid. Heat the mixture under reflux in an oil bath for 1 hour with continuous stirring. Once the hour is up, allow the mixture to cool to room temperature and filter to remove any precipitate that may have formed. The filtrate should be added to 300 mL of water, and the precipitate collected through vacuum filtration. The filtrate should be neutralized with ammonium hydroxide, and the second batch of solid collected. Combine the two batches of precipitate and recrystallize them from aqueous EtOH. After purifying the material, record the yield and melting point (m.p.).
B) Preparation of photochromic dimer
To carry out the experiment, first, dissolve potassium hydroxide KOH in 100 mL of 95 % EtOH by heating and add 1.5 g of 2,4,5‐triphenylimidazole. After complete dissolution, transfer the mixture to a 1 L beaker containing a large magnetic stir bar and cool it to 5 ºC with stirring in an ice-water bath. In an addition funnel clamped securely over the beaker, prepare a solution of potassium ferricyanide K3[Fe(CN)6] in 450 mL of water. Add this solution to the vigorously stirred ethanolic mixture at such a rate that the temperature of the reaction does not exceed 10 ºC. During the addition, a violet color develops initially, which later turns into a light-grey precipitate. After completion of the addition, filter off the precipitate with vacuum, wash thoroughly with water, and dry the precipitate on the filter. Record the yield, appearance, and melting point (m.p.) of the product and store it in the dark.
Photochromic test
Grind a small quantity of the product in a mortar and observe the color change. Dissolve 20 mg of the product in 30 mL of toluene, stopper, and store the mixture in the dark. After the solution becomes colorless or nearly so, expose it to strong sunlight or irradiate it with a bright tungsten light and note the effect. Finally, return the solution to the dark and observe it after about a day.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Microwave-Mediated Synthesis of Lophine: Developing a Mechanism To Explain a Product
R. David Crouch, Jessica L. Howard, Jennifer L. Zile, and Kathryn H. Barker
Journal of Chemical Education 2006 83 (11), 1658
DOI: 10.1021/ed083p1658 - Synthesis, photophysical and computational studies of two lophine derivatives with electron-rich substituents in the 2-position
Terianne Hamada, Tammy Le, Matthew J. Voegtle, Bryan Doyle, Jarred Rimby, Ralph Isovitsch
Journal of Molecular Structure 1130 (2017) 284-290
DOI: 10.1016/j.molstruc.2016.10.050 - Oxidation of Triarylimidazoles. Structures of the Photochromic and Piezochromic Dimers of Triarylimidazyl Radicals1
D. M. White and J. Sonnenberg
Journal of the American Chemical Society 1966 88 (16), 3825-3829
DOI: 10.1021/ja00968a027