Written by J.A Dobado | Last Updated on April 22, 2024
Objective
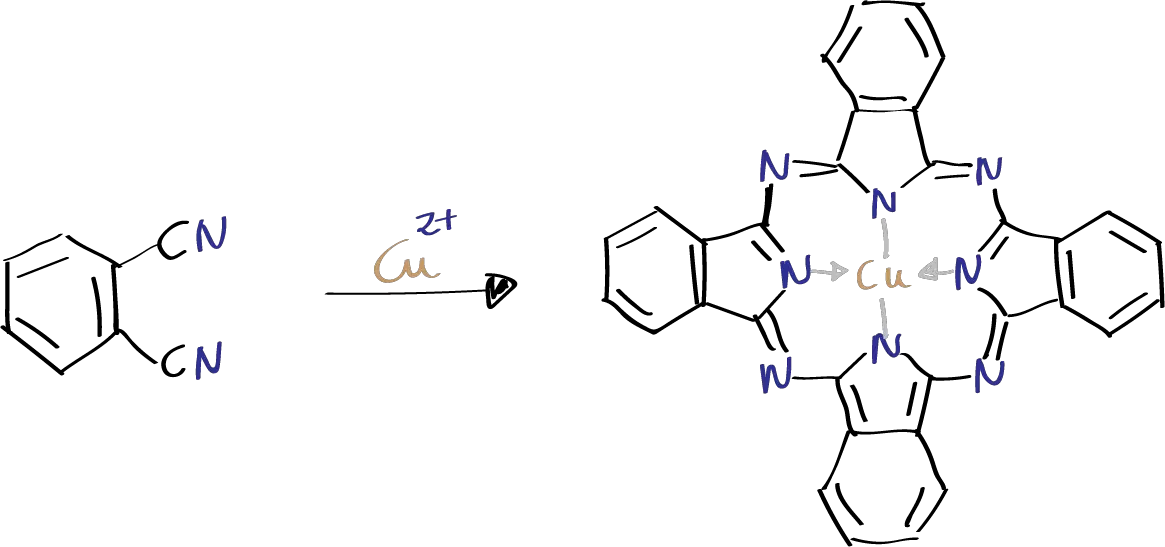
The objective of the experiment is to obtain a copper phthalocyanine (macrocycle with four isoindole units linked together by nitrogen atoms bonded to copper) by combining four molecules of phthalonitrile in the presence of a metal salt.
Background
Phthalocyanines are a class of highly stable blue pigments that can contain various coordinated metals. The copper-based compound is widely used as a blue coloring agent in paints and printing inks. The complex ring system is similar to naturally occurring porphyrins, including examples like haem and chlorophyll.
Its high stability and colorfastness make it a popular choice as a pigment in printing inks, paints, and plastics. Copper phthalocyanine is also used in the production of currency notes and security documents due to its resistance to forgery.
Copper phthalocyanine has also found its way into the field of electronics. It is used as an organic semiconductor material in the production of solar cells, light-emitting diodes (LEDs), and field-effect transistors (FETs). The molecule’s ability to conduct electricity makes it a promising material for the development of organic electronic devices.
Copper phthalocyanine is easily synthesized by combining four molecules of phthalonitrile in the presence of a metal salt.
Experimental procedure
To synthesize copper phthalocyanine, gather the following materials: phthalonitrile (3.2 g, 25 mmol), anhydrous copper(II) chloride (2.0 g, 16 mmol), 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) (2.5 g, 20 mmol), and bis(2‐methoxyethyl) ether (diglyme) (10 mL). Place them in a 100 mL round-bottomed flask and heat until the solvent boils (at around 160 °C). Once boiling, continue to heat under reflux for approximately 2 hours. The refluxing process ensures that the reaction occurs at a constant temperature and prevents loss of materials through evaporation.
After 2 hours, cool the flask and pour the contents into a beaker of water. Briefly bring the water to a boil to dissolve any unreacted copper compounds, then cool it down. The base DBN can be removed from the reaction mixture by acidification. To do this, add a few drops of dilute hydrochloric acid to the reaction mixture until the pH reaches around 2-3. The solution will become acidic, which will cause the base to precipitate out. Vacuum filter the solution to remove the base, and collect the blue copper phthalocyanine powder.
If the copper phthalocyanine is obtained as a brown solid, it can be purified by dissolving the finely ground product in concentrated sulfuric acid H2SO4 (approximately 5 mL acid per gram of product). Leave the mixture to sit for around 30 minutes, then carefully pour the acid solution onto crushed ice (100 g) in a beaker. Allow the blue flocculent precipitate to coalesce, then collect it by vacuum filtration. Finally, wash the product thoroughly with hot water and dry it at 100 °C.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1,5-Diazabicyclo[4.3.0]non-5-ene | 124.18 | 95-98 | - | 1.005 |
| Diglime | 134.17 | -64 | 162 | 0.937 |
| Copper(II) chloride | 134.45 | 498 | decomposes | 3.386 |
| 1,5-Diazabicyclo[4.3.0]non-5-ene | 124.18 | 95-98 | - | 1.005 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| HCl | 36.46 | -30 | >100 | 1.200 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1,5-Diazabicyclo[4.3.0]non-5-ene |  |
| Diglime |  |
| Copper(II) chloride |   |
| 1,5-Diazabicyclo[4.3.0]non-5-ene |  |
| H2SO4 |  |
| HCl |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1,5-Diazabicyclo[4.3.0]non-5-ene | SGUVLZREKBPKCE-UHFFFAOYSA-N |
| Diglime | SBZXBUIDTXKZTM-UHFFFAOYSA-N |
| Copper(II) chloride | ORTQZVOHEJQUHG-UHFFFAOYSA-L |
| 1,5-Diazabicyclo[4.3.0]non-5-ene | SGUVLZREKBPKCE-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- McKeown, N. B. (2000). Phthalocyanine-containing polymers. J. Mater. Chem., 10(9), 1979-1995. DOI: 10.1039/B000793P
- Phthalocyanine compounds
Frank H. Moser and Arthur L. Thomas
Journal of Chemical Education 1964 41 (5), 245
DOI: 10.1021/ed041p245