Objective
To perform ether synthesis using phase-transfer catalyst in a water-basic solution.
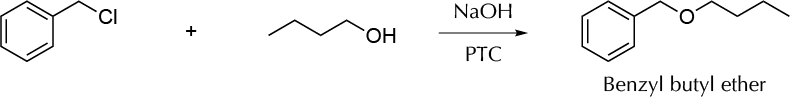
Background
In the usual Williamson ether synthesis, a primary alkyl halide reacts with an alkoside. The reaction conditions must be rigorous, paying special attention to the anhydrous solvent. Even a trace of water can spoil the reaction. However, the Williamson ether synthesis may be carried out under milder conditions by employing a phase-transfer catalyst. This procedure is highly efficient and can be used with conventional solvents (without being anhydrous). In this experiment, benzyl butyl ether is synthesized from benzylchloride and butanol with a phase-transfer catalyst such as tetrabutylammonium hydrogen sulfate.
Reaction mechanism
In a round-bottom flask, make a 50 % solution of NaOH by dissolving 20 g of NaOH (0.5 mol) in water (20 ml). Cool to r.t. and add 7.4 g (9.2 ml, 0.1 mol) of 1-butanol. A thick, white slurry will form, followed by benzyl chloride (15.2 g, 13.8 ml, 0.12 mol) and tetrabutylammonium hydrogen sulfate (1.7 g, 0.005 mol). Fit with a reflux condenser, heat the mixture in a water bath to 75 ºC for 15 min with stirring. The crude is cooled to room temperature and transferred to a separtory funnel. The final product is extracted with methylene chloride CH2Cl2 (2 × 30 ml). Organic extracts are dried over anhydrous MgSO4. When dried, remove the desiccant by gravity filtration on a tared round-bottom flask. The solvent is eliminated under vacuum (rotary evaporator) to give a liquid. The process can provide good yield (50-90 %, average 67 %). Benzyl butyl ether can be purified by vacuum distillation (m.p. = 111-112 ºC, 23 mmHg).
| DANGER! “Benzyl chloride has a strong, irritating odor that causes tearing of the eyes, can be harmful when inhaled, and may be absorbed through the skin.” |
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzyl chloride | 126.58 | 177-181 | - | 1.1 |
| Butan-1-ol | 74.12 | -90 | 116-118 | 0.810 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| MgSO4 | 120.37 | 1124 | - | 1.070 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| Tetrabutylammonium hydrogensulfate | 339.53 | - | 169-171 | 1.01 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzyl chloride |    |
| Butan-1-ol |    |
| CH2Cl2 |  |
| MgSO4 | Non-hazardous |
| NaOH |  |
| Tetrabutylammonium hydrogensulfate |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzyl chloride | KCXMKQUNVWSEMD-UHFFFAOYSA-N |
| Butan-1-ol | LRHPLDYGYMQRHN-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| MgSO4 | CSNNHWWHGAXBCP-UHFFFAOYSA-L |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| Tetrabutylammonium hydrogensulfate | OHLITOUHJLRUQJ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- J. W. Hill and J. Corredor, An Ether Synthesis Using Phase Transfer Catalysis, Journal of Chemical Education 57 (1980), no. 11, 822, DOI: 10.1021/ed057p822