Written by J.A Dobado | Last Updated on April 22, 2024
Objective
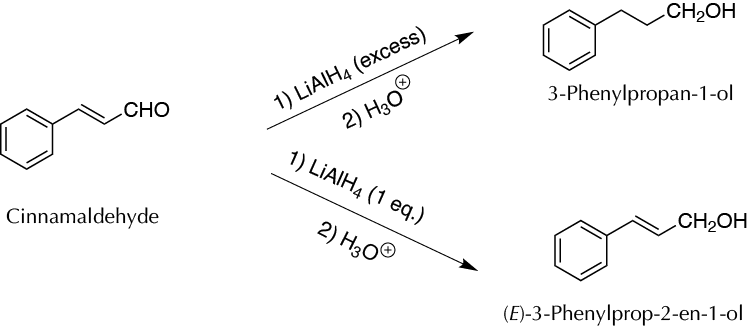
To transform of cinnamaldehyde (2E)-3-phenylprop-2-enal) into 3-phenyl-2-propen-1-ol and 3-phenyl-propan-1-ol, using a single reagent, the LiAlH4, depending on the relative quantities and the addition order, in an inert atmosphere.
Background
The functional group interconversion (FGI) reactions are among the most common synthetic processes. In this experiment, the conversion of cinnamic aldehyde is performed by reduction with LiAlH4 into two different products, depending upon the order of addition of reactants.
In a first experiment, the reaction is carried out by adding a solution of the aldehyde in anhydrous tetrahydrofuran (THF), over a suspension of LiAlH4 in THF, also anhydrous (direct addition). Under these conditions, the simultaneous reduction of the carbonyl group and the double bond occurs because the reducing agent is in excess in the medium in which the reaction occurs.
In a second experiment, the addition of a suspension of LiAlH4 in dry diethyl ether to a solution of the aldehyde in the same solvent (inverse addition) is performed. With this protocol, only the reduction of the aldehyde group occurs, leaving the double bond unchanged.
Because of the reactivity of LiAlH4, the reaction should be performed under anhydrous conditions in an inert atmosphere. For the correct performance of this experiment, the glassware should be dry, the solvents should have undergone a previous treatment to remove traces of moisture, and the whole experiment should be conducted in an inert atmosphere with argon or nitrogen. On the other hand, when the experiment is finished, but before discarding waste, the remaining LiAlH4 reactive must be handled properly to avoid accidents.
Experimental procedure
A) Cinnamaldehyde reduction to alcohol:
In this process, LiAlH4 in excess is added to a solution of cinnamaldehyde, and both functional groups (double bond and carbonyl group) are reduced. In a dry vial, weigh 2.9 g of LiAlH4 and transfer it, using approximately 20 ml of THF, to a dry three-neck flask and set up an addition funnel and a reflux condenser with a bubbler. Couple a gas inlet to the third neck of the flask, and inlet a gently stream of inert gas (nitrogen or argon). Gentle reflux and add to the addition funnel a solution of 5 g of cinnamaldehyde in 50 ml of tetrahydrofuran (THF), and add dropwise to the flask while the reflux is maintained. When the reaction is complete (about 15 min, follow the reaction by TLC, using CH2Cl2 as the eluent), cool the flask in an ice bath and carefully add 12 ml of a saturated sodium sulfate to destroy the excess hydride, and after stirring a few minutes add another solution of 10% H2SO4 (95 ml). Transfer to a separatory funnel, separate the two layers, and extract the aqueous layer with ether (4 × 30 ml). Collect the ether extracts, dry over anhydrous sodium sulfate, and remove the solvent on a rotary evaporator. Vacuum distill the resulting oil: hydrocinnamic alcohol (b.p. = 120–121 ºC/13 mm Hg).
B) Cinnamaldehyde reduction to cinnamic alcohol:
In this procedure, an equimolar amount of LiAlH4 is used only to reduce the carbonyl group to alcohol. Add to the flask a solution of 10 g of cinnamaldehyde and 25 ml of anhydrous ether. Weigh 0.72 g of LiAlH4, and add to addition funnel suspended in 50 ml of anhydrous ether. Place in an ice-water bath, and start adding the suspension LiAlH4 during an interval of 30 min, ensuring that the temperature does not exceed 10 ºC (the reflux condenser can be replace with an adapter with a thermometer). Check the reaction progress by TLC. When the reaction is complete (disappearance of cinnamaldehyde in the TLC plate), add 3 ml of water (to destroy possible reagent excess), and then add 25 ml of H2SO4 10%. Transfer to a separatory funnel, decant, and extract twice with 50 ml of ether. Collect the ether extracts, dry over anhydrous sodium sulfate, and remove the solvent in the rotary evaporator. Vacuum distill the oil produced, cinnamic alcohol (b.p. = 139 ºC/14 mm Hg). The product can be recrystallized (m.p. = 33 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Cinnamaldehyde | 132.16 | -7.5 | 248 | 1.050 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| LiAlH4 | 37.95 | 125 | - | 0.920 |
| Tetrahydrofuran | 72.11 | -108.0 | 65-67 | 0.89 |
| N2 | 28.01 | -210 | -196 | 0.970 |
| Argon | 39.95 | -189.2 | -185.7 | - |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| Cinnamyl alcohol | 134.18 | 30-33 | 250 | |
| 3-Phenylpropan-1-ol | 136.19 | -18 | 119-121 | 1.001 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Cinnamaldehyde |  |
| Na2SO4 | Non-hazardous |
| LiAlH4 |   |
| Tetrahydrofuran |   |
| N2 |  |
| Argon | Non-hazardous |
| H2SO4 |  |
| Diethyl ether |   |
| Cinnamyl alcohol |  |
| 3-Phenylpropan-1-ol |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Cinnamaldehyde | KJPRLNWUNMBNBZ-QPJJXVBHSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| LiAlH4 | BJKLPLABXHXMIM-UHFFFAOYSA-N |
| Tetrahydrofuran | WYURNTSHIVDZCO-UHFFFAOYSA-N |
| N2 | IJGRMHOSHXDMSA-UHFFFAOYSA-N |
| Argon | XKRFYHLGVUSROY-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| Cinnamyl alcohol | OOCCDEMITAIZTP-QPJJXVBHSA-N |
| 3-Phenylpropan-1-ol | VAJVDSVGBWFCLW-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145