Written by J.A Dobado | Last Updated on April 22, 2024
Objective
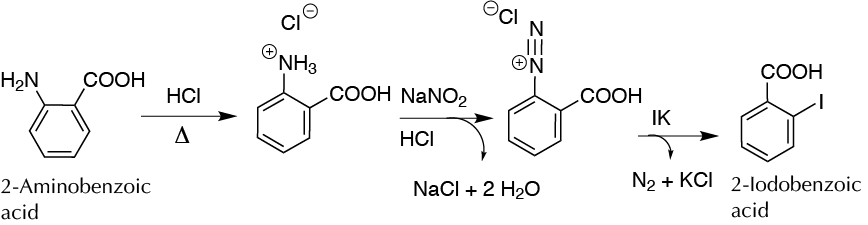
To produce at microscale iodobenzoic acid by the formation of an intermediate diazonium salt (Sandmeyer reaction).
Background
The Sandmeyer reaction is a versatile synthetic tool by which an amino group on an aromatic ring is replaced with a wide range of substituents by converting an amino group attached to an aromatic ring into a diazonium salt that can be transformed into several functional groups. In this experiment, the 2-iodobenzoic acid is synthesized from 2-aminobenzoic acid by this reaction.
Microscale experimental procedure
In a 5 ml conical vial provided with a spin vane, add 137 mg of 2-aminobenzoic acid (1 mmol), 1 ml of deionized water, and 250 μl of concentrated HCl. Stir the mixture until complete dissolution of the 2-aminobenzoic acid, gently heating if necessary. Place the vial with a septum in an ice bath, and slowly add with a syringe and a needle a solution of 75 mg of NaNO2 dissolved in 300 μl of water with stirring. Wash the syringe with 100 μl of additional water to the reaction crude.
Stir the mixture for 5 min while keeping it cold, and then add, again with the help of a clean syringe and a needle, 170 mg of potassium iodide KI dissolved in 250 μl of water. Observe that a brown product is formed that partly precipitates.
After the addition is finished, the vial is quickly uncovered and fit to an air condenser to prevent spillage of the foam formed. Remove the ice bath, and then stir the reaction mixture for 5 min at r.t. Then introduce the conical vial into a water bath and heat at 40 ºC for 10 min. At this point, gas (nitrogen) vigorously bubbles and a dark precipitate appears. Then allow the temperature of the bath to rise to 80 ºC for another 10 min, considering at this point that the reaction is finished.
Cool the vial with an ice bath, and add several mg of sodium sulfite to destroy the iodine that may be present. Filter the resulting solid under vacuum and wash with 1 ml of deionized water. Recrystallize the solid from the water using a Craig tube.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaNO2 | 69.00 | 271 | 320 | 2.164 |
| KI | 166.00 | 681 | 1,330 | 3.130 |
| Anthranilic acid | 137.14 | 144-148 | - | - |
| 2-Iodobenzoic acid | 248.02 | 160-162 | - | - |
| Na2SO3 | 126.04 | >500 | - | 2.630 |
| NaCl | 58.44 | 801 | 1,413 | 2.165 |
| KCl | 74.55 | 770 | 1,500 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| HCl |   |
| NaNO2 |    |
| KI |  |
| Anthranilic acid |  |
| 2-Iodobenzoic acid |   |
| Na2SO3 | Non-hazardous |
| NaCl | Non-hazardous |
| KCl | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaNO2 | LPXPTNMVRIOKMN-UHFFFAOYSA-M |
| KI | NLKNQRATVPKPDG-UHFFFAOYSA-M |
| Anthranilic acid | RWZYAGGXGHYGMB-UHFFFAOYSA-N |
| 2-Iodobenzoic acid | CJNZAXGUTKBIHP-UHFFFAOYSA-N |
| Na2SO3 | GEHJYWRUCIMESM-UHFFFAOYSA-L |
| NaCl | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
| KCl | WCUXLLCKKVVCTQ-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145