Written by J.A Dobado | Last Updated on April 22, 2024
Objective
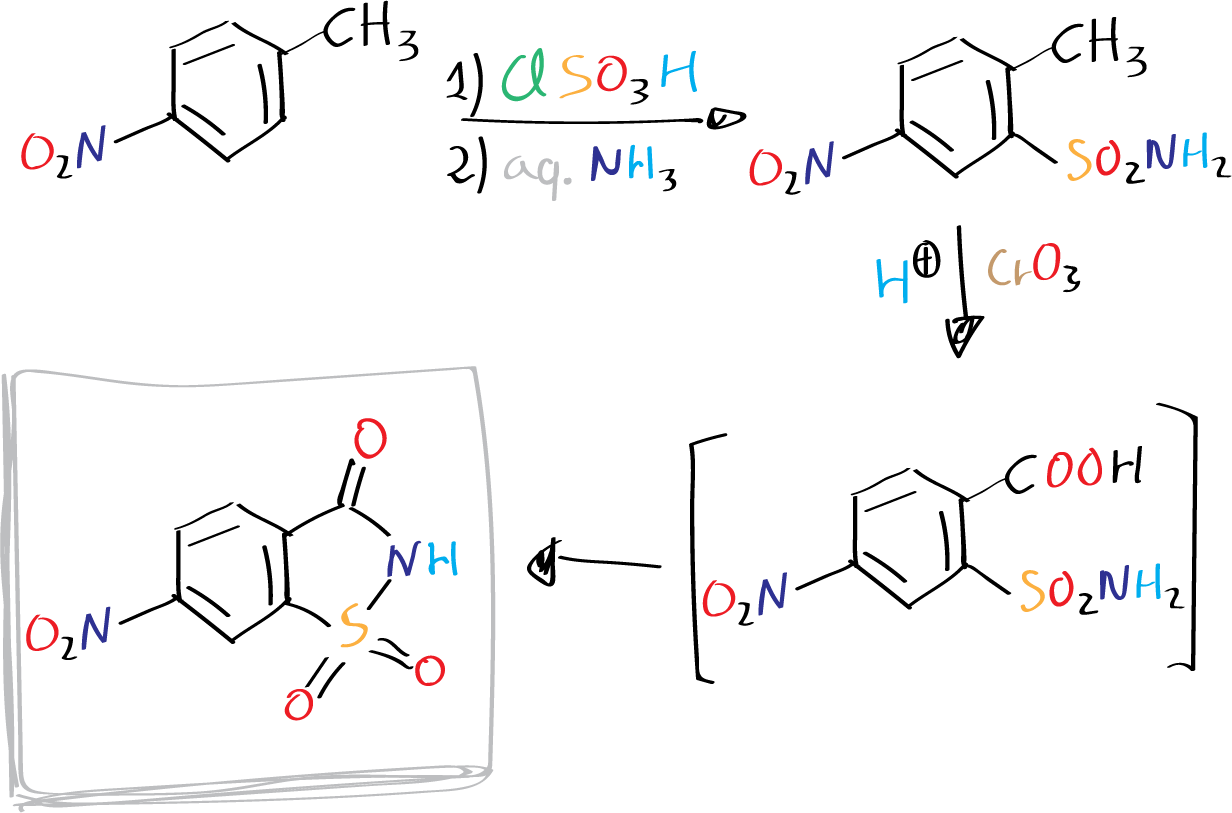
The objective is to synthesize a sweetener derivative, specifically the 6-nitro derivative of saccharin, using a two-step process involving 4-nitrotoluene and oxidation with CrO3.
Background
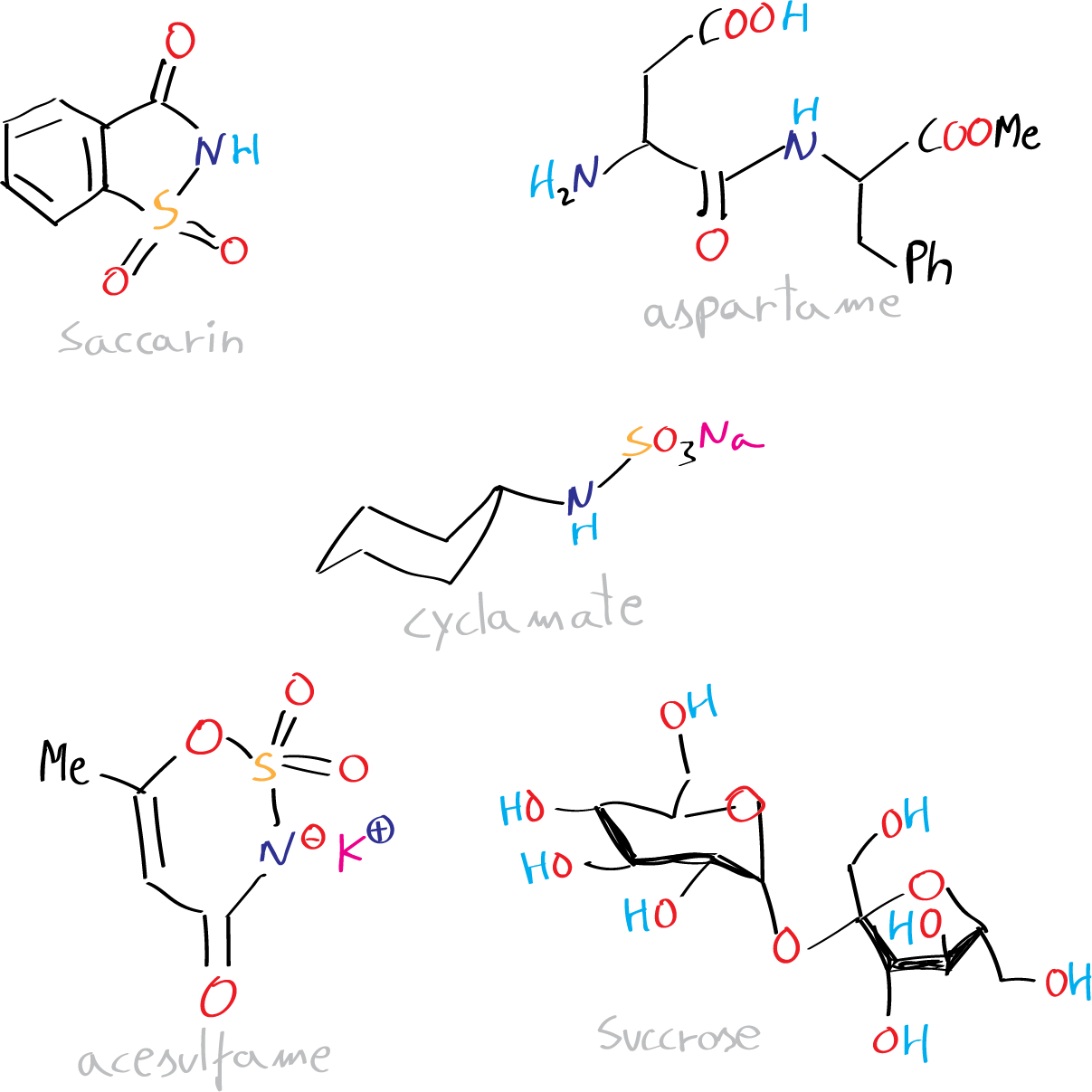
Foodstuffs derive their sweetness from either natural sugars or added artificial sweeteners. Natural sugars, such as sucrose, glucose, and fructose, have desirable taste qualities, but come with drawbacks such as high energy content and the need for high concentrations in processed and convenience foods. To address these concerns, artificial sweeteners, which are often hundreds of times sweeter than sucrose, have found wide application. While they have varying chemical structures, controversy surrounding cyclamate (now banned) and saccharin (banned in certain countries) led to the development of newer sweeteners, including aspartame and acesulfame.
This experiment explores the chemistry of aromatic compounds through the preparation of the 6-nitro derivative of saccharin from 4-nitrotoluene. The experiment involves several critical steps. The initial stage includes the chlorosulfonation of 4-nitrotoluene, producing 4-nitrotoluene-2-sulfonyl chloride, which is then transformed into the corresponding sulfonamide by reacting with aqueous ammonia. The subsequent step involves oxidation of the toluene methyl group utilizing chromium(VI) oxide in sulfuric acid, resulting in the o-sulfonamidobenzoic acid. The final step involves a spontaneous cyclization of the o-sulfonamidobenzoic acid, forming the 6-nitrosaccharin.
Experimental procedure
A) Preparation of 4‐nitrotoluene‐2‐sulfonamide
In a 25mL round-bottomed flask, add the 4-nitrotoluene, followed by careful addition of chlorosulfonic acid. Fit the flask with a reflux condenser and heat under reflux for 30 minutes. Cool the mixture in an ice bath, then pour it into a beaker containing approximately 100 g of ice, stirring vigorously with a glass rod. Transfer the mixture to a separatory funnel and extract with 2×20 mL of diethyl ether. Combine the ether extracts and transfer them to a 250 mL beaker. Rapidly stir the diethyl ether solution and gradually add 20 mL of ammonia solution, continuing to stir until a light-brown solid forms. Collect the solid through vacuum filtration, wash it thoroughly with 20 mL of cold diethyl ether followed by 40 mL of cold water. Dry the solid briefly, using vacuum pump, and then recrystallize it from hot water. Record the yield and melting point (m.p.) of the product after a single recrystallization.
B) Preparation of 6‐nitrosaccharin
Add 2.15 g (10 mmol) of dry 4-nitrotoluene-2-sulfonamide to a 100 mL beaker containing 12 mL of concentrated sulfuric acid, H2SO4. Heat the mixture gently to 65 °C, stirring it continuously. In small portions, add chromium(VI) oxide to the stirred solution at a rate that maintains the temperature between 65 and 70 ºC. Do not add the oxidant (CrO3) unless the temperature is at least 65 °C. The addition should take 15-30 minutes, during which the mixture turns green and viscous. Once complete, stir the mixture for an additional 10 minutes at 65-70 ºC before cooling it in an ice bath. Pour the reaction mixture into a beaker containing 50 mL of cold water and stir for a few minutes until a solid forms. Collect the solid by vacuum filtration, and wash it well with cold water before drying it through suction at the pump for a few minutes. Recrystallize the product from hot water and record the yield and melting point (m.p.).
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Preparation of 6-aminosaccharin
Norman C. Rose and Sanford Rome
Journal of Chemical Education 1970 47 (9), 649
DOI: 10.1021/ed047p649