Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To synthethise two kind of insect pheromones one using Grignard reagent to yield an alcohol, and the other (a ketone) through the oxidation of the alcohol using chromium(VI).
Background
Insect pheromones are chemical signals produced by insects to communicate with members of their own species. These chemical signals are used to transmit information about a variety of behaviors such as mating, marking of territories, aggregation, alarm, and recognition of nestmates. The chemical compounds used as pheromones are usually volatile and can be detected by other insects at low concentrations over long distances. Insect pheromones are widely used in agriculture and pest management to disrupt insect mating behaviors or attract them to traps for monitoring or control.
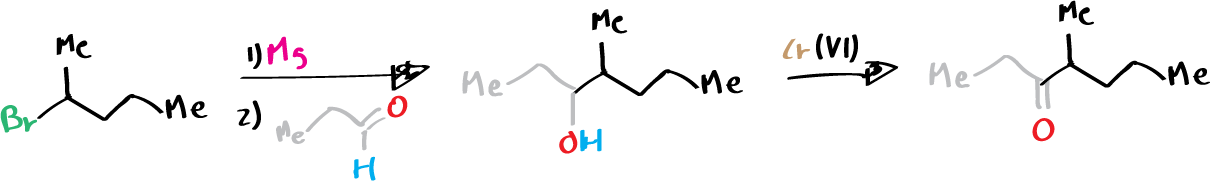
The European elm bark beetle, Scolytus multistriatus, is a major contributor to the spread of Dutch elm disease, and it uses a combination of three aggregation pheromones to communicate. One of these pheromones is 4-methylheptan-3-ol, which can be easily synthesized by adding the Grignard reagent derived from 2-bromopentane to propanal. Additionally, the corresponding ketone, 4-methylheptan-3-one, also functions as a pheromone and serves as an alarm signal for various ant species, such as the harvester ant, Pogonomyrmex barbatus, and the Texas leafcutter ant, Atta texana. This secondary alcohol can be synthesized through the oxidation of the corresponding Grignard reagent using chromium(VI).
Experimental procedure
A) Preparation of (±)-4‐methylheptan‐3‐ol
To set up the reaction, take a 100 mL three-neck flask and attach a 25 mL addition funnel, a reflux condenser protected by a drying tube, and a magnetic stir bar. Into the flask, add 15 mL of dry diethyl ether and magnesium, and stopper the third neck. Add a small amount of the 2-bromopentane solution in dry diethyl ether to the magnesium and stir the mixture to initiate the formation of the organometallic reagent. Gradually add the remaining bromide solution over 15 minutes while stirring continuously, and continue stirring for an additional 10 minutes once the addition is complete. Meanwhile, prepare a solution of propanal in 10 mL of dry diethyl ether and place it in the addition funnel. Add this solution dropwise to the stirred Grignard solution and continue the stirring for another 15 minutes after the addition is complete. Gradually add 10 mL of water followed by 10 mL of dilute hydrochloric acid (10%) until all the inorganic salts dissolve. Decant the mixture from the remaining magnesium into a separatory funnel, separate the ether layer, and wash it with 10 mL of 5 % sodium hydroxide NaOH solution. Collect the ether layer and dry it over MgSO4. Gravity filter off the drying agent, and evaporate the filtrate using a rotary evaporator. Transfer the residue to a small distillation set and distill it at atmospheric pressure, collecting the fraction boiling in the range of 150-165 ºC. Finally, record the yield and IR spectrum of the obtained product.
B) Preparation of (±)-4‐methylheptan‐3‐one
Place 35 mL distilled water and a magnetic stirrer bar in a 100 mL Erlenmeyer flask. Clamp the flask in an ice bath, start the stirrer and add the concentrated sulfuric acid, H2SO4. Add the sodium dichromate and stir the mixture until a clear orange solution is obtained. Continue to stir the solution and add 5.0 g (38 mmol) 4‐methylheptan‐3‐ol in small portions over about 10 minutes; the colour of the reaction mixture should gradually change to green. Stir the mixture for a further 10 minutes and then transfer it to a 100 mL separatory funnel. Add 200 mL diethyl ether, shake the funnel and separate the organic layer. Wash the ether layer with 3×20 mL portions of 5 % sodium hydroxide NaOH solution and dry it over MgSO4. Filter off the drying agent, evaporate the filtrate on the rotary evaporator and distil the residue from a small distillation set at atmospheric pressure, collecting the fraction boiling in the range 155-160 °C. Record the yield and IR spectrum of your product.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Synthesis of 4-methyl-3-heptanol and 4-methyl-3-heptanone. Two easily synthesized insect pheromones
Robert M. Einterz, Jay W. Ponder, and Ronald S. Lenox
Journal of Chemical Education 1977 54 (6), 382
DOI: 10.1021/ed054p382