Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To perform one-pot synthesis of a tetrahydropyrimidinone by a three-component reaction (Biginelli reaction).
Background
The Biginelli reaction, first described in 1893, is a three-component reaction between an aldehyde, a β-ketoester, and urea to yield in a one-pot procedure dihydropyrimidones. This reaction is a quick and easy method to synthesize heterocycles, compounds with a great potential for pharmaceutical application.
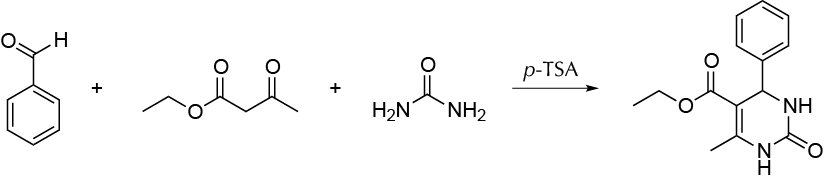
Experimental procedure
In a mortar, grind together equivalent amounts of benzaldehyde (0.5 mol), ethyl acetoacetate (0.5 mol), and urea (0.5 mol) with a small amount of p-toluenesulfonic acid (as an acid catalyst), for about 3-5 min to give a light-yellow solid mass that will be mostly the target tetrahydropyrimidinone. Wash the crude product with cold water to remove the color, and purify by recrystallization from acetone/EtOH (m.p. = 208–210 ºC, estimated yield 94 %).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Ethyl acetoacetate | 130.14 | -43 | 181 | 1.029 |
| Benzaldehyde | 106.12 | -26 | 178-179 | 1.044 |
| Urea | 60.06 | 132-135 | - | 1.335 |
| p-Toluenesulfonic acid | 172.2 | 106-107 | - | 1.240 |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Ethyl acetoacetate |  |
| Benzaldehyde |  |
| Urea |  |
| p-Toluenesulfonic acid |   |
| Acetone |   |
| EtOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Ethyl acetoacetate | XYIBRDXRRQCHLP-UHFFFAOYSA-N |
| Benzaldehyde | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| Urea | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
| p-Toluenesulfonic acid | JOXIMZWYDAKGHI-UHFFFAOYSA-N |
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A. K. Bose, S. Pednekar, S. N. Ganguly, G. Chakraborty, and M. S. Manhas, A simplified green chemistry approach to the Biginelli reaction using Grindstone Chemistry, Tetrahedron Lett. 45 (2004), no. 45, 8351–8353, DOI: 10.1016/j.tetlet.2004.09.064