Written by J.A Dobado | Last Updated on April 22, 2024
Objective
Recycled polyethylene terephthalate (PET) by a depolymerization reaction and use of the product for the synthesis of a diester of high added value.
Background
A large number of synthetic polymers are materials that are usually obtained from monomers originating from the petrochemical industry.
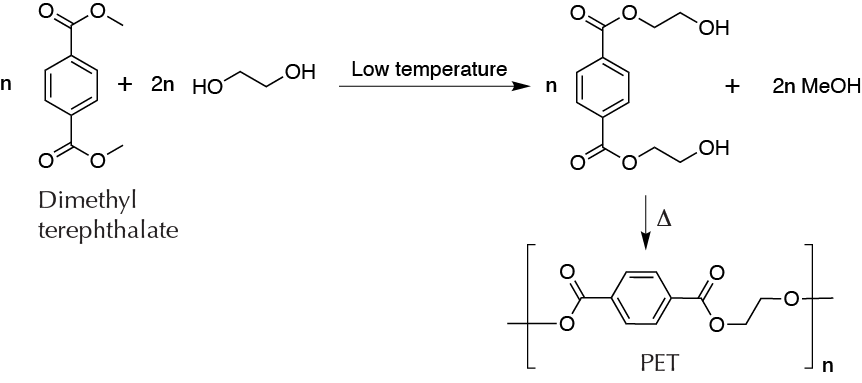
Once the lifetimecycle of objects made from polymers has expired, they pose a serious environmental problem, because many of them have a strong resistance to biodegradation. Thus they can remain in landfills without significant alterations for years.Today, the technologies developed for recycling different types of plastic polymers are of great interest for yielding other products or materials as alternatives to burning or incineration. PET is a thermoplastic polyester that is widely used for the manufacture of packaging for beverages or food products, textiles, etc. It is produced from dimethyl terephthalate and ethylene glycol. De-polymerizing PET yields the starting monomer (dimethyl terephthalate), which can be again used to synthesize new molecules of PET.
Experimental procedure
A) Preparation of PET samples
From bottles of soda or mineral water previously cleaned without labels, using scissors, cut plastic into pieces under 3 × 3 cm to obtain a mass of approximately 5 g.
B) Hydrolysis of PET samples
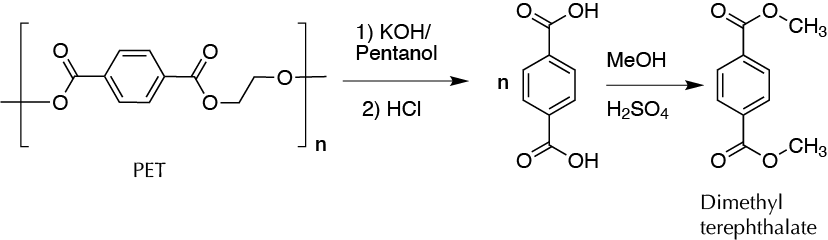
To a 100 ml round-bottom flask, add 35 ml of pentan-1-ol (mixture of isomers or pentan-1-ol), 5.0 g of PET (0.052 mol equivalent ester), and 4.4 g of KOH (0.079 mol). Reflux the mixture with magnetic stirring. While at first the polymer does not dissolve, shortly after dissolution a dense white emulsion forms. If magnetic stirring is stopped by the viscosity of the reaction crude, add more solvent in portions of 5 ml. Once the stirring resumes, maintain the reflux for 1.5 h. Afterward, cool the reaction to r.t., and add 25 ml of water so that the white solid (terephthalate potassium salt) is dissolved. Remove any remaining suspended solid by vacuum filtration as the water/pentan-1-ol mixture filters very slowly by gravity. Transfer the filtrate to a separatory funnel. Decant the aqueous (lower) layer and wash the organic (top) with another 25 ml of water. Collect in a beaker both aqueous layers, and add diluted HCl very slowly with magnetic stirring to neutrality. Once the terephthalic acid has precipitated, filter the crystals under vacuum and wash with portions of 5 ml of acetone. Weigh, calculate yield, and store for the next step.
C) Producing dimethyl terephthalate
In a round-bottom flask, add 2.7 g of terephthalic acid and 20 ml MeOH. Cool the mixture in an ice bath and slowly add with a dropper 3 ml of concentrated H2SO4. Add a stir bar into the flask and reflux (using a drying tube) for 90 min. Cool the flask contents to r.t. Filter the resulting solid under vacuum and wash with two portions of 10 ml of CH2Cl2 to dissolve all the dimethyl terephthalate that may have crystallized.
Transfer the filtrate together with the CH2Cl2 portions to a separatory funnel, and wash successively with 25 ml of water, twice with 25 ml of 10% NaOH, and finally with 25 ml of brine. Dry the organic layer (bottom) over anhydrous sodium sulfate, and remove the drying agent by gravity filtration. Transfer the dried solution to a round-bottom flask, remove the solvent under reduced pressure (rotary evaporator), weigh, and calculate the yield. Finally, transfer the solid to a Petri dish for storage
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| Pentan-1-ol | 88.15 | -78 | 136-138 | 0.811 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| KOH | 56.11 | 361 | 1,320 | 2.044 |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
| PET (C10H12O6)n | - | 250-255 | - | 1.680 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| Terephthalic acid | 166.13 | >300 | - | 1.500 |
| Dimethyl terephthalate | 194.18 | 139-141 | 282 | 1.350 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetone |   |
| Pentan-1-ol |   |
| CH2Cl2 |  |
| NaOH |  |
| KOH |   |
| MeOH |    |
| PET (C10H12O6)n | Non-hazardous |
| H2SO4 |  |
| HCl |   |
| Na2SO4 | Non-hazardous |
| Terephthalic acid |  |
| Dimethyl terephthalate | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| Pentan-1-ol | AMQJEAYHLZJPGS-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| KOH | KWYUFKZDYYNOTN-UHFFFAOYSA-M |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
| PET (C10H12O6)n | |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| Terephthalic acid | KKEYFWRCBNTPAC-UHFFFAOYSA-N |
| Dimethyl terephthalate | WOZVHXUHUFLZGK-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- D. Kaufman, G. Wright, R. Kroemer, and J. Engel, New compounds from old plastics: recycling PET plastics via depolymerization. An activity for the undergraduate organic lab, Journal of Chemical Education 76 (1999), no. 11, 1525, DOI: 10.1021/ed076p1525