Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The purpose of this experiment is to obtain alkenes from phosphonium salts and carbonyl compounds by means of the Wittig reaction.
Background
The Wittig reaction is named after its discoverer Georg Wittig (Nobel Prize winner in 1979) and consists of obtaining alkenes from phosphonium salts and carbonyl compounds. The carbon-carbon double bond C=C is selectively formed on carbon with a carbonyl group C=O.
The hydrogens of the α-position carbons of phosphonium salts are acidic due to resonance stabilization of the carbanionic species when treated with a base.
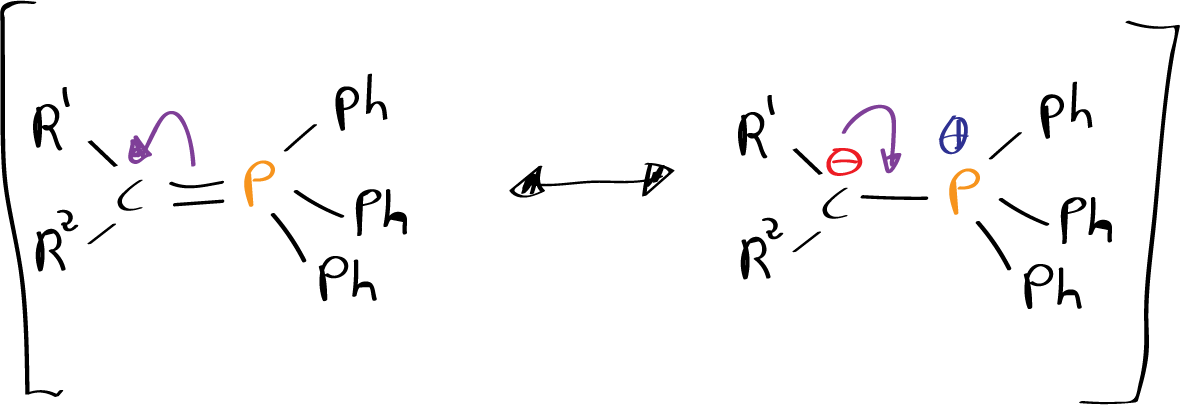
Phosphonium salts are prepared by reaction of trisubstituted phosphines, usually triphenylphosphine with alkyl halides. Deprotonation usually requires strongly basic conditions, although in the case of stabilized ilides (with electron-accepting groups by resonance) milder bases can be used.
The reaction can be considered to begin by nucleophilic attack on the carbonyl carbon to give a dipolar betaine, followed by the loss of phosphine oxide, generating an alkene.
In this fragmentation a cyclic oxaphosphetane is involved, which can be formally considered as a [2+2] cycloaddition product of the ylide on the carbonyl compound.
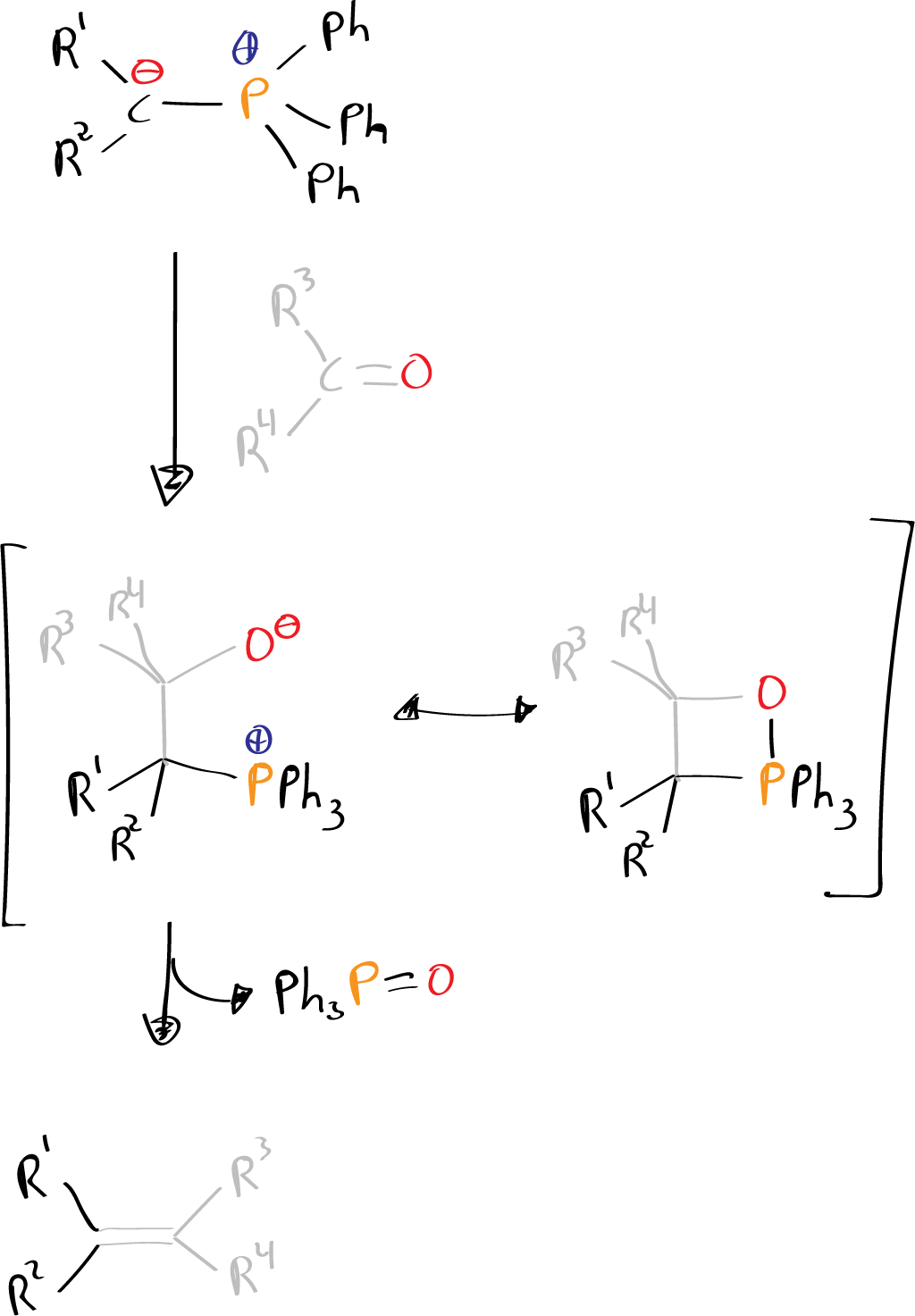
Due to the strength of the P=O bond, the final extrusion step is thermodynamically highly favored, allowing the synthesis of alkenes not available by other methods, such as tensioned alkenes, terminal alkenes or systems with unconjugated double bonds.
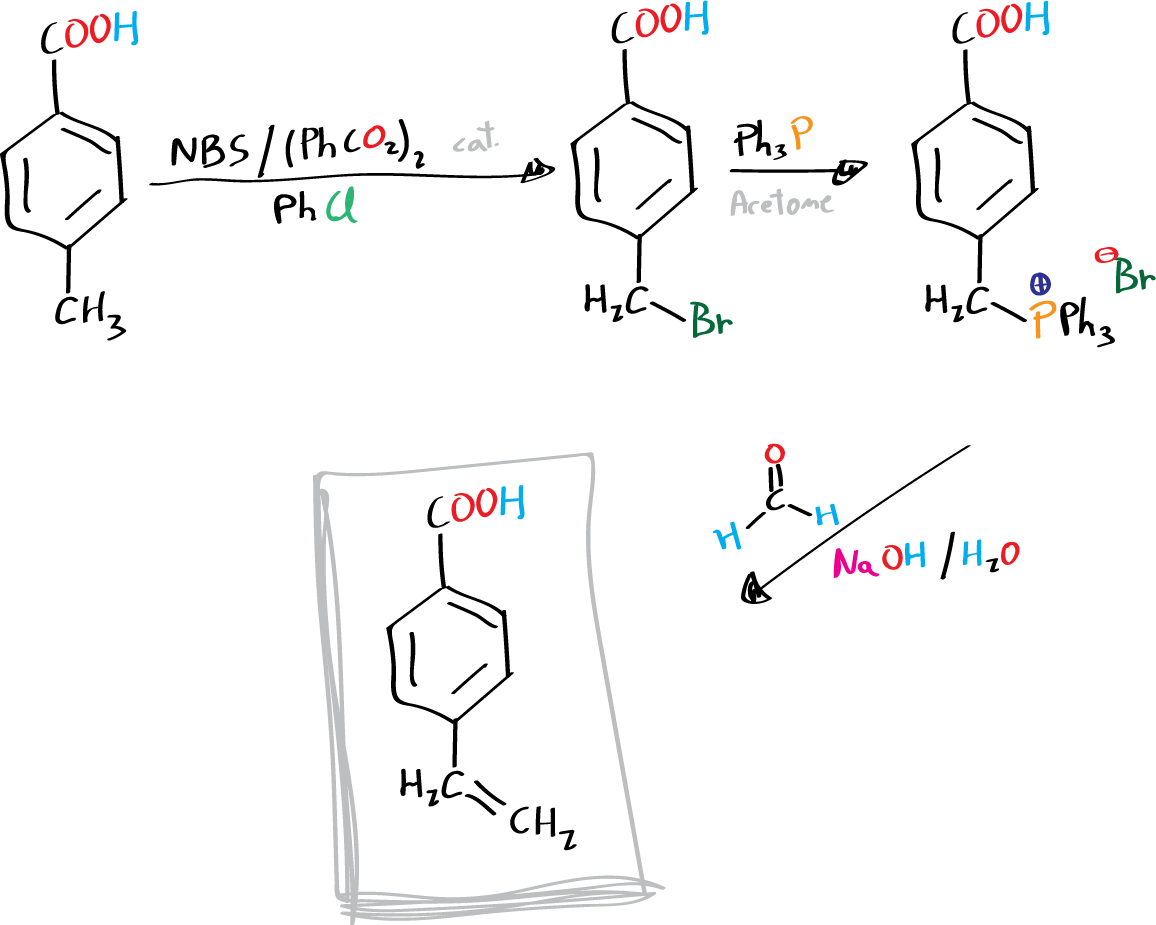
In this experiment, the ilide is generated in the presence of a large excess of aldehyde. The ilide is stabilized by the electron-accepting effect of the carboxylic acid (aromatic ring) and is also resistant to hydrolysis. The phosphonium salt is prepared from 4-bromomethylbenzoic acid, which is non-volatile and therefore relatively safe to handle, although somewhat irritating.
The 4-bromomethylbenzoic acid is generated from the corresponding 4-methylbenzoic acid by a radical reaction using benzoyl peroxide as initiator and the compound N-bromosuccinimide as bromine source.
Experimental procedure
Preparation of 4-bromomethylbenzoic acid
Place 2.7 g of 4-methylbenzoic acid and 3.6 g of N-bromosuccinimide, in a 100 ml round bottom flask. Add benzoyl peroxide (0.2 g) taking care that it does not adhere to the flask ground glass. Finally, to add 25 ml of chlorobenzene, dragging the solid that could have remained in the inner walls of the flask.
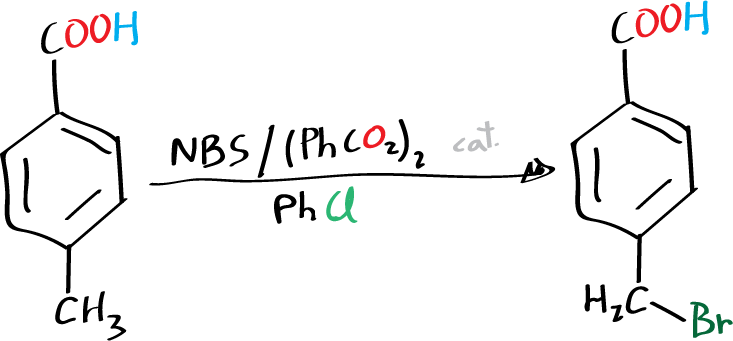
Then the mixture is heated gently at reflux for 1 h with magnetic stirring. After this period the flask is cooled in an ice bath for 10 min.
The precipitate of the cold reaction crude is filtered off under vacuum. The residue is washed with petroleum ether (hexane) (3 x 10 ml) and transferred to a beaker. 50 ml of water is added and stirred for 10 min to dissolve the succinimide. It is filtered, again under vacuum and the precipitate is washed successively with water (2 x 10 ml) and petroleum ether (hexane) (2 x 10 ml).
The product is dried by passing an air stream through the Büchner. It is used in the next step.
| DANGER ! “Benzoyl peroxide is an oxidizing agent and prone to explode both by percussion and by heating or friction. HANDLE WITH EXTREME CAUTION!“ |
Preparation of 4-carboxybenzyltriphenylphenoxyphosphonium bromide
Dissolve 4-bromomethylbenzoic acid (4.3 g, 20 mmol) and triphenylphosphine (5.2 g, 20 mmol) in 60 ml acetone in a 100 ml round bottom flask. Reflux the mixture for 45 min. After this period, cool the reaction crude.
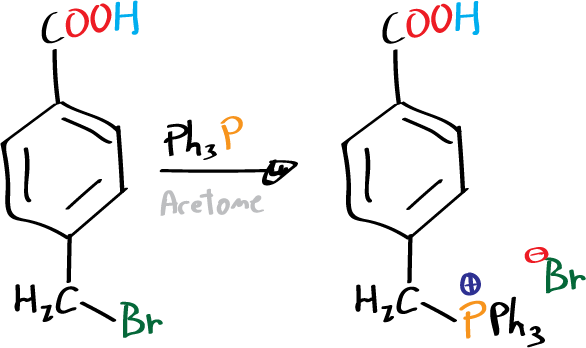
The cold reaction crude is filtered under vacuum to obtain the precipitated phosphonium salt. Wash the solid with diethyl ether and dry it by air stream in the Büchner. Weigh the dry product and use it in the next step.
Preparation of 4-vinylbenzoic acid
Place 4-carboxybenzyltriphenylphosphonium bromide (3.76 g, 8 mmol), aqueous formaldehyde (32 ml) and 15 ml of water in a 250 ml Erlenmeyer flask, equipped with magnetic stirring. Shake vigorously, holding the Erlenmeyer with a clamp and add a solution of sodium hydroxide (2.5 g in 15 ml of water). Shake the mixture for 45 min.
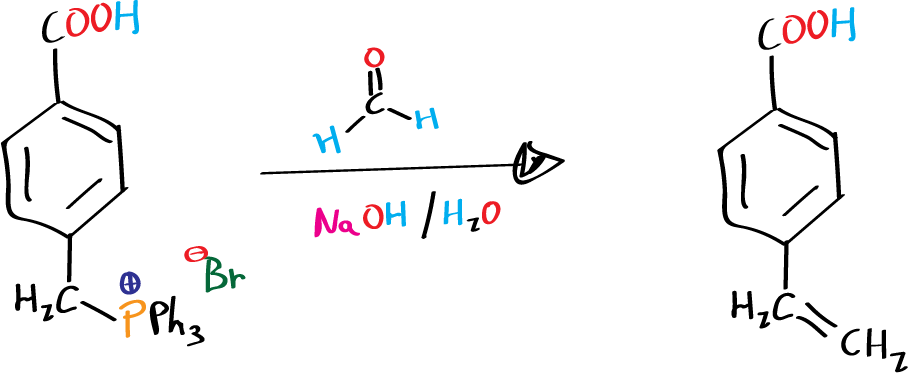
The triphenylphosphine oxide precipitates in the reaction crude and is filtered under vacuum and washed with water (solid which is discarded). Both the filtrate and the wash waters are acidified with concentrated HCl yielding a solid corresponding to 4-vinylbenzoic acid. The product is filtered under vacuum and recrystallized from aqueous EtOH.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 4-(Bromomethyl)benzoic acid | 215.04 | 224-229 | - | 1.69 |
| 4-Vinylbenzoic acid | 148.16 | 142-144 | - | - |
| Acetone | 58.08 | -94 | 56 | 0.791 |
| Benzoyl peroxide | 242.23 | 105 | - | - |
| Chlorobenzene | 112.56 | -45 | 132 | 1.106 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| N-Bromosuccimide | 177.98 | 175-180 | - | - |
| Triphenylphosphine | 262.29 | 79-81 | 377 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 4-(Bromomethyl)benzoic acid |  |
| 4-Vinylbenzoic acid |  |
| Acetone |   |
| Benzoyl peroxide |   |
| Chlorobenzene |    |
| Diethyl ether |   |
| HCl |   |
| Hexane |     |
| N-Bromosuccimide |   |
| Triphenylphosphine |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 4-(Bromomethyl)benzoic acid | CQQSQBRPAJSTFB-UHFFFAOYSA-N |
| 4-Vinylbenzoic acid | IRQWEODKXLDORP-UHFFFAOYSA-N |
| Acetone | CSCPPACGZOOCGX-UHFFFAOYSA-N |
| Benzoyl peroxide | OMPJBNCRMGITSC-UHFFFAOYSA-N |
| Chlorobenzene | MVPPADPHJFYWMZ-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| N-Bromosuccimide | PCLIMKBDDGJMGD-UHFFFAOYSA-N |
| Triphenylphosphine | RIOQSEWOXXDEQQ-UHFFFAOYSA-N |
Video on Wittig reaction for obtaining 4-vinylbenzoic acid
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- S. H. Leung and S. A. Angel, Solvent-free wittig reaction: a green organic chemistry laboratory experiment, Journal of Chemical Education 81 (2004), no. 10, 1492, DOI: 10.1021/ed081p1492