Written by J.A Dobado | Last Updated on April 22, 2024
Aromatic hydrocarbons
Although formally the structure of benzene would be that corresponding to 1,3,5-cyclohexatriene (i.e. corresponding to a conjugated polyene) its properties do not correspond to those of a polyene.
For example, it does not undergo addition reactions under any of the conditions specified in alkene and diene reactions, its heat of hydrogenation is abnormally low (about 30 kcal/mol less than expected), its geometry indicates that all bonds are equivalent, in NMR the protons resonate at abnormally low fields with respect to those of alkenes, etc.
Some of these features could be explained by considering the structure of benzene as a resonance hybrid between several resonance structures:
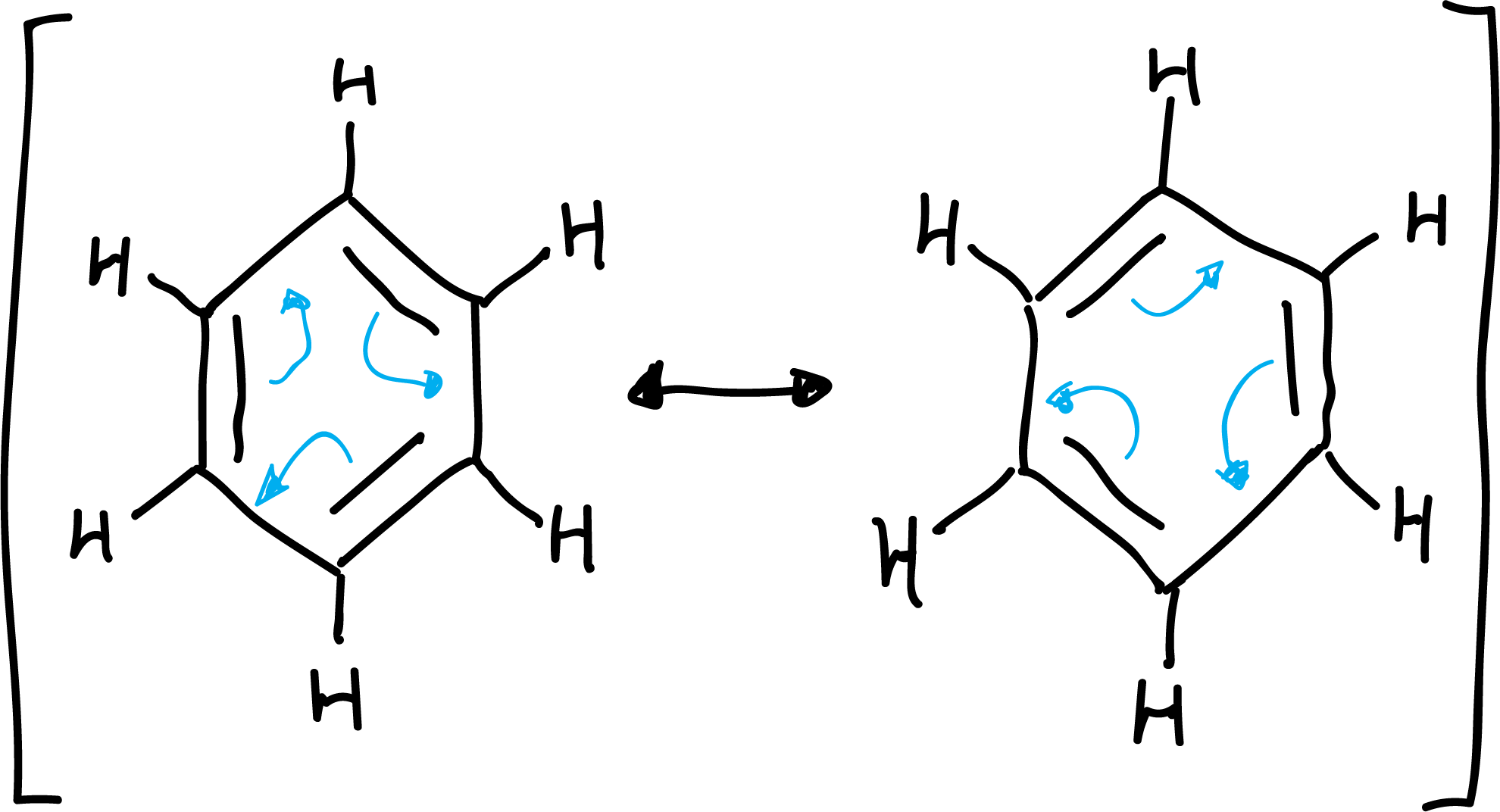
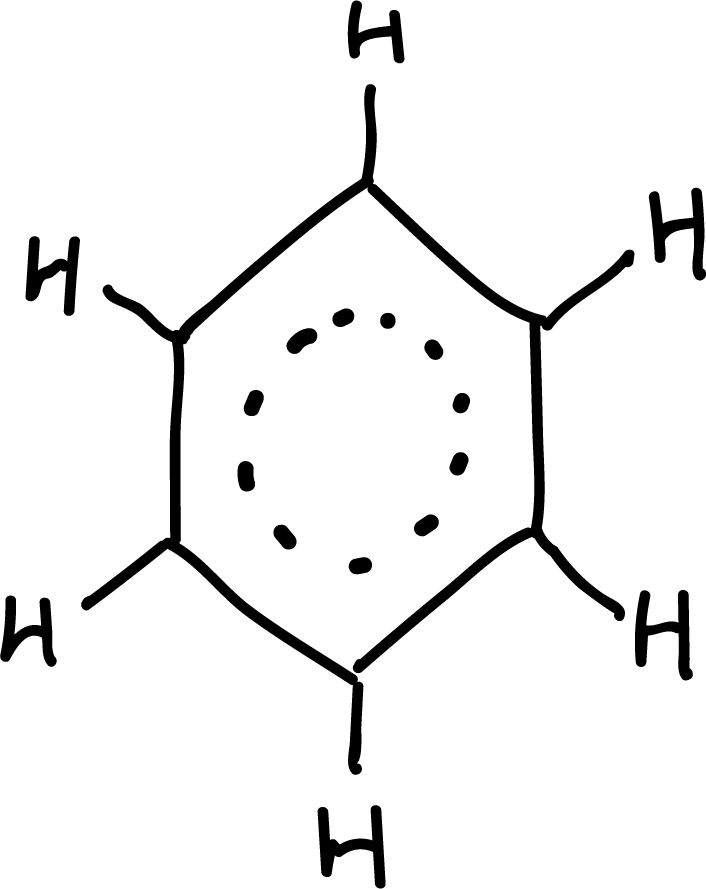
Aromaticity
The property responsible for all these exceptional properties is called aromaticity and is not only present in benzene.
For a structure to be aromatic, it must in principle meet three conditions:
- Be a cyclic conjugated polyene system.
- Be a flat structure.
- Hückel’s rule must be fulfilled: the number of electrons involved in the conjugate system must correspond to the formula:
electrons = 4n + 2 for aromatic systems or 4n for antiaromatic systems.
where n is an integer (0, 1, 2, etc). Some examples of aromatic compounds are illustrated below:
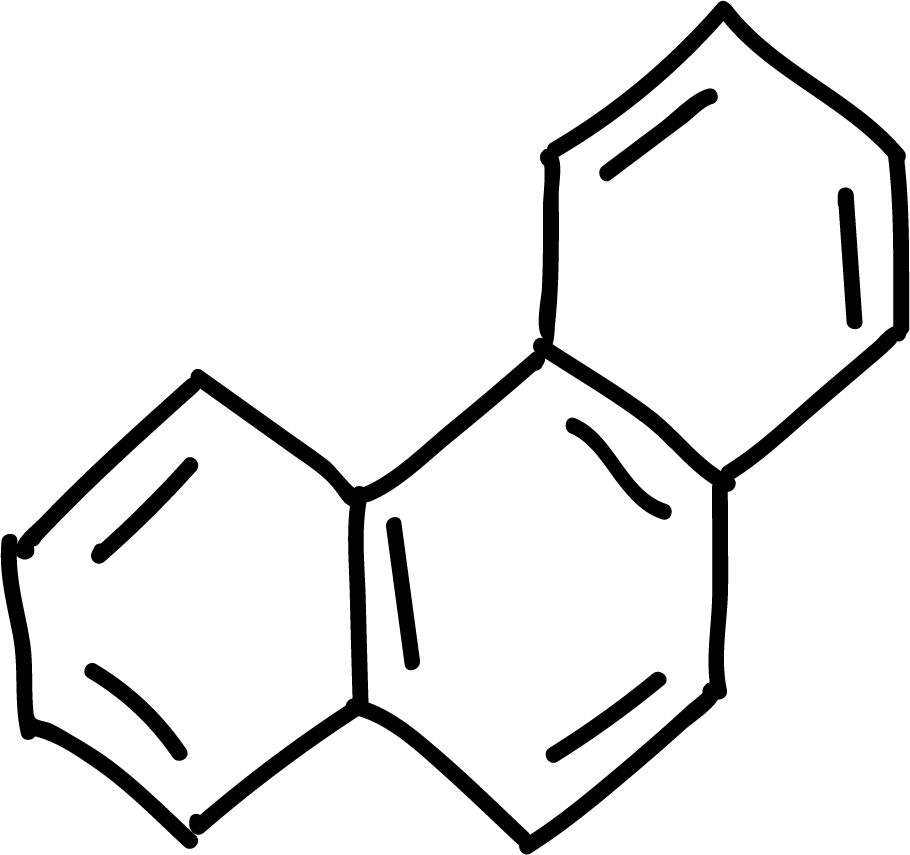
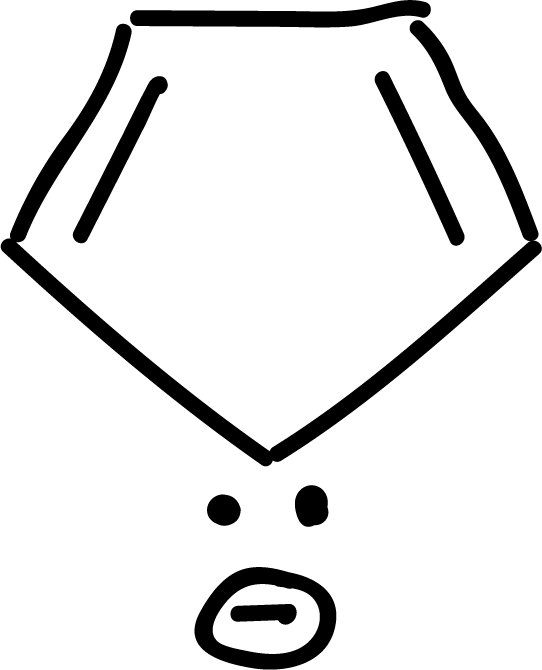
and other anti-aromatics:
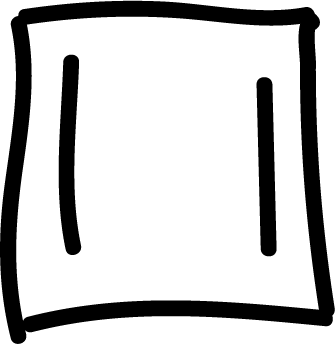
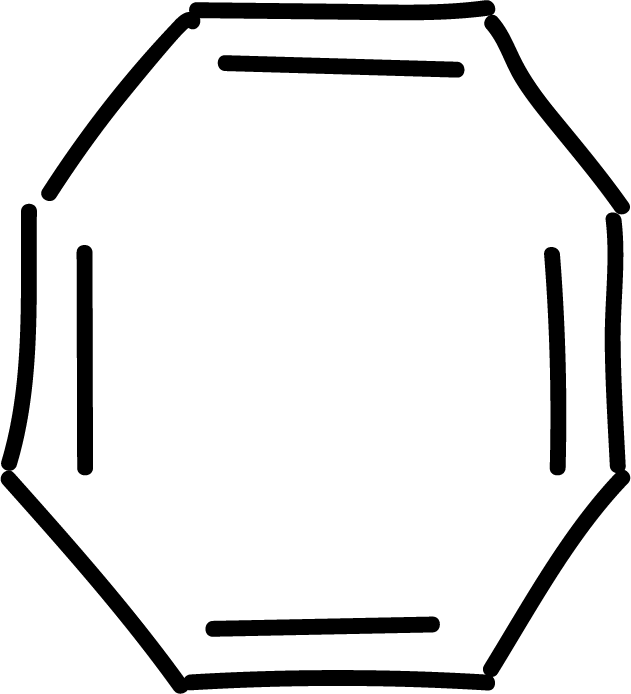
Electrophilic aromatic substitution (SEAr) in benzene
Electrophilic aromatic substitution reactions (SEAr) allow the replacement of a hydrogen from an aromatic ring by an electrophilic reagent, E⊕. For a description of the reaction mechanism see link.
Reaction conditions
Depending on the nature of the electrophile, a wide variety of aromatic derivatives can be obtained. Generally, the reactions are catalyzed with Lewis acid or mineral acids.
| Reaction type | Reactives | Electrophile (product) |
| Halogenation | X2 / FeX3 | X⊕ (Ar-X) |
| Nitration | HNO3 / H2SO4 | NO2⊕ (Ar-NO2) |
| Sulfonation | H2SO4 / SO3 | SO3 (Ar-SO3H) |
| Alkylation | R-Cl / AlCl3 o R-OH / H⊕ o C=C / H⊕ | R⊕ (Ar-R) |
| Acylation | R-COCl o (RCO)2O / H⊕ | RCO⊕ (Ar-COR) |
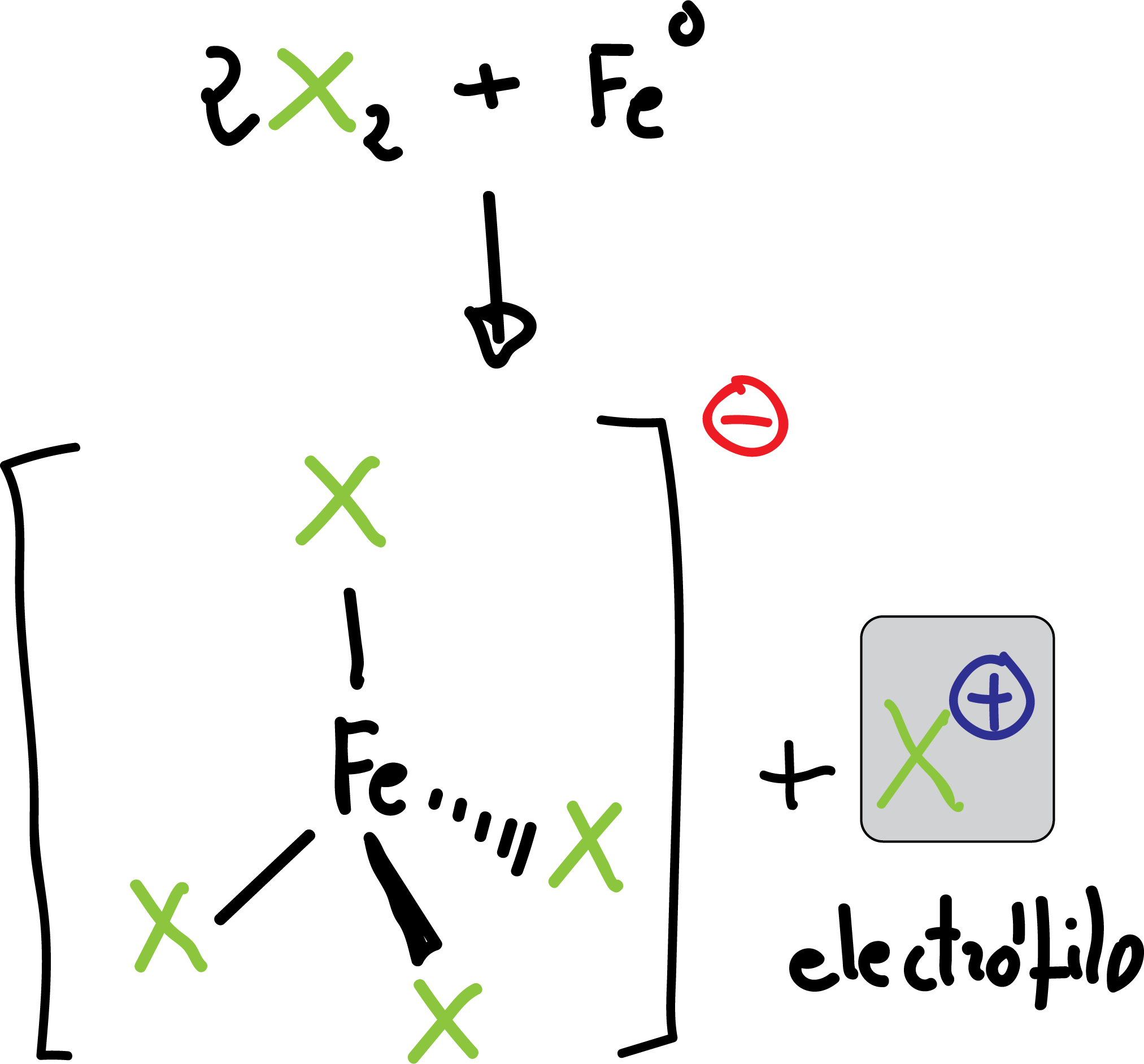
Halogenation
The reaction is carried out by treating the aromatic compound with the halogen in the presence of a Lewis acid (Feº which is transformed into FeX3 in situ by reaction with the halogen).
<
FeX3 acts as a Lewis acid, releasing the electrophile X⊕.
The reaction is applicable for the case of Cl⊖ and Br⊖. To introduce I⊖ or F⊖ other methods are used which will be discussed later (see Synthesis and reactivity of diazonium salts).
Nitration
In this case the electrophile, ⊕NO2 (nitronium ion), is obtained by loss of a water molecule from HNO3, by the action of sulfuric acid H2SO4, which is a good dehydrating agent. H2SO4 acts as an acid against HNO3 which acts as a base (a much weaker acid than H2SO4). This is an acid-base equilibrium reaction.
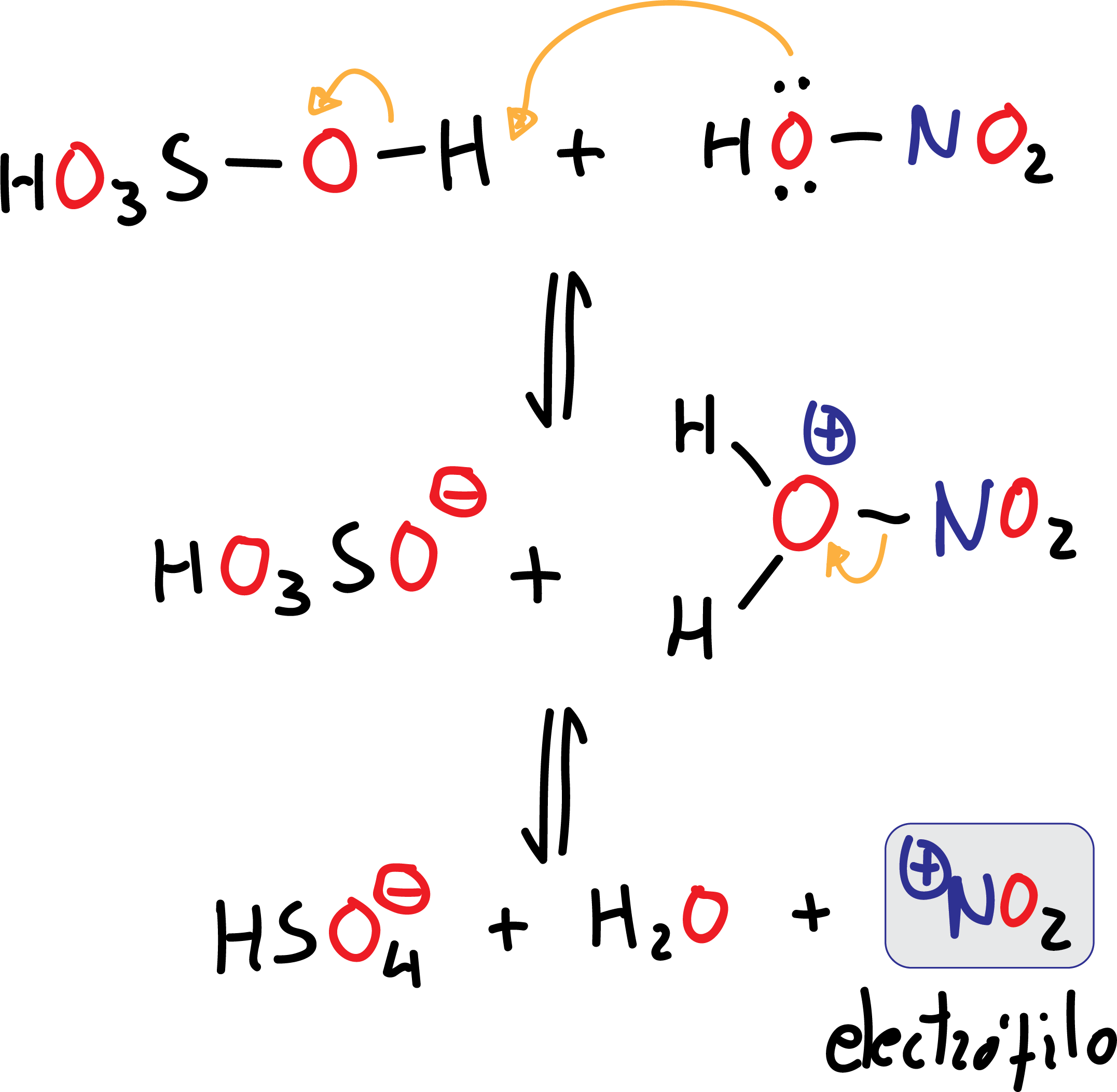
The nitronium ion ⊕NO2 is widely known as salts such as nitronium fluoroborate (NO2BF4) or nitronium perchlorate (NO2ClO4) exist. These dissolved stable nitronium salts (in nitromethane or acetic acid) are able to slightly nitrate aromatic compounds at room temperature and in high yields.
Sulfonation
The electrophile is SO3, which is obtained by loss of a water molecule from the sulfuric acid H2SO4 itself when it is heated.
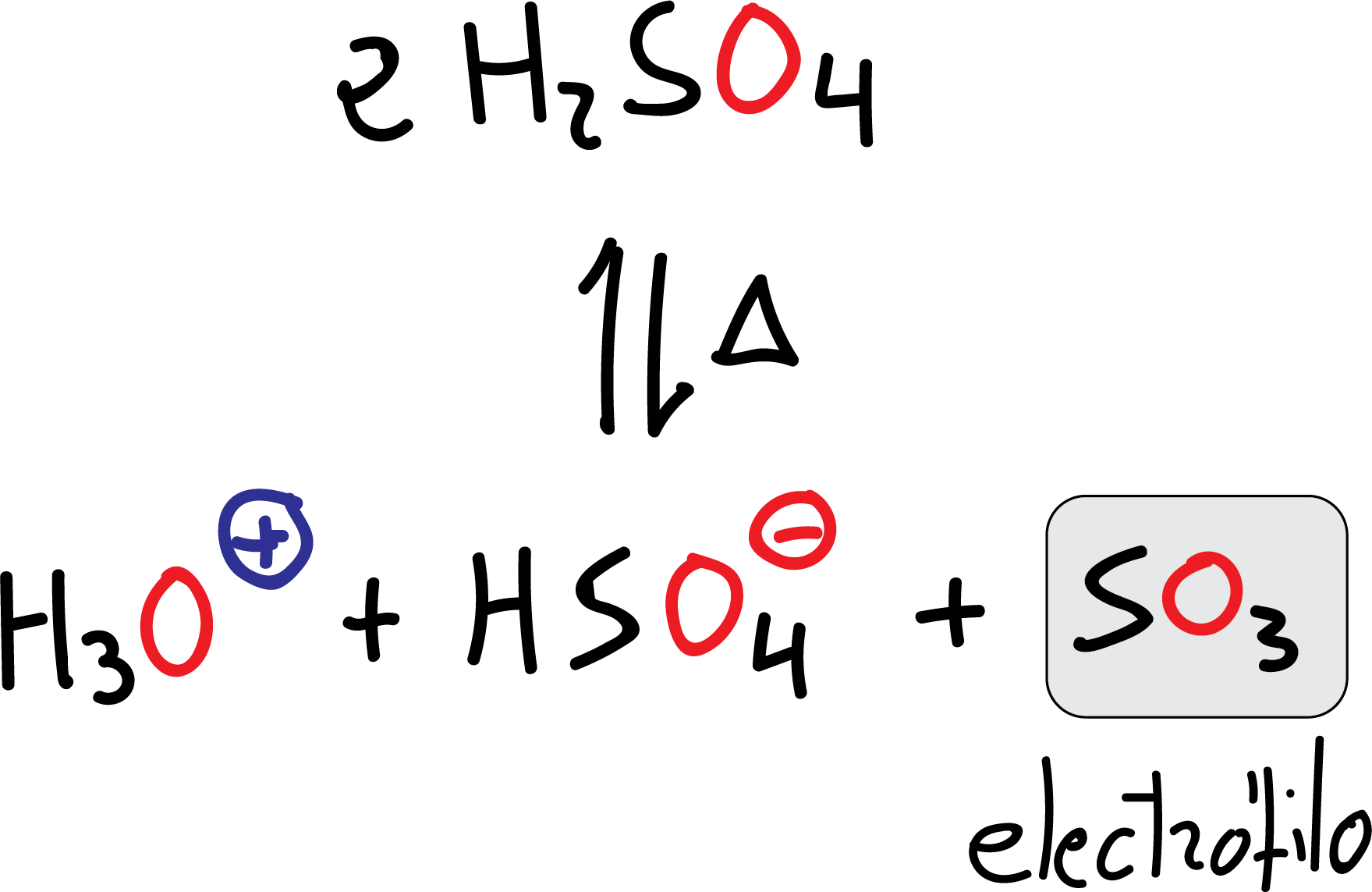
Although SO3 is present in concentrations of about 20 % in the so-called fuming sulfuric acid, which is the reagent used with benzene or weakly reactive substrates. Unlike other electrophiles, the SO3 molecule is neutral and has no formal charge.
The mechanism of the sulfonation reaction in benzene is as follows:
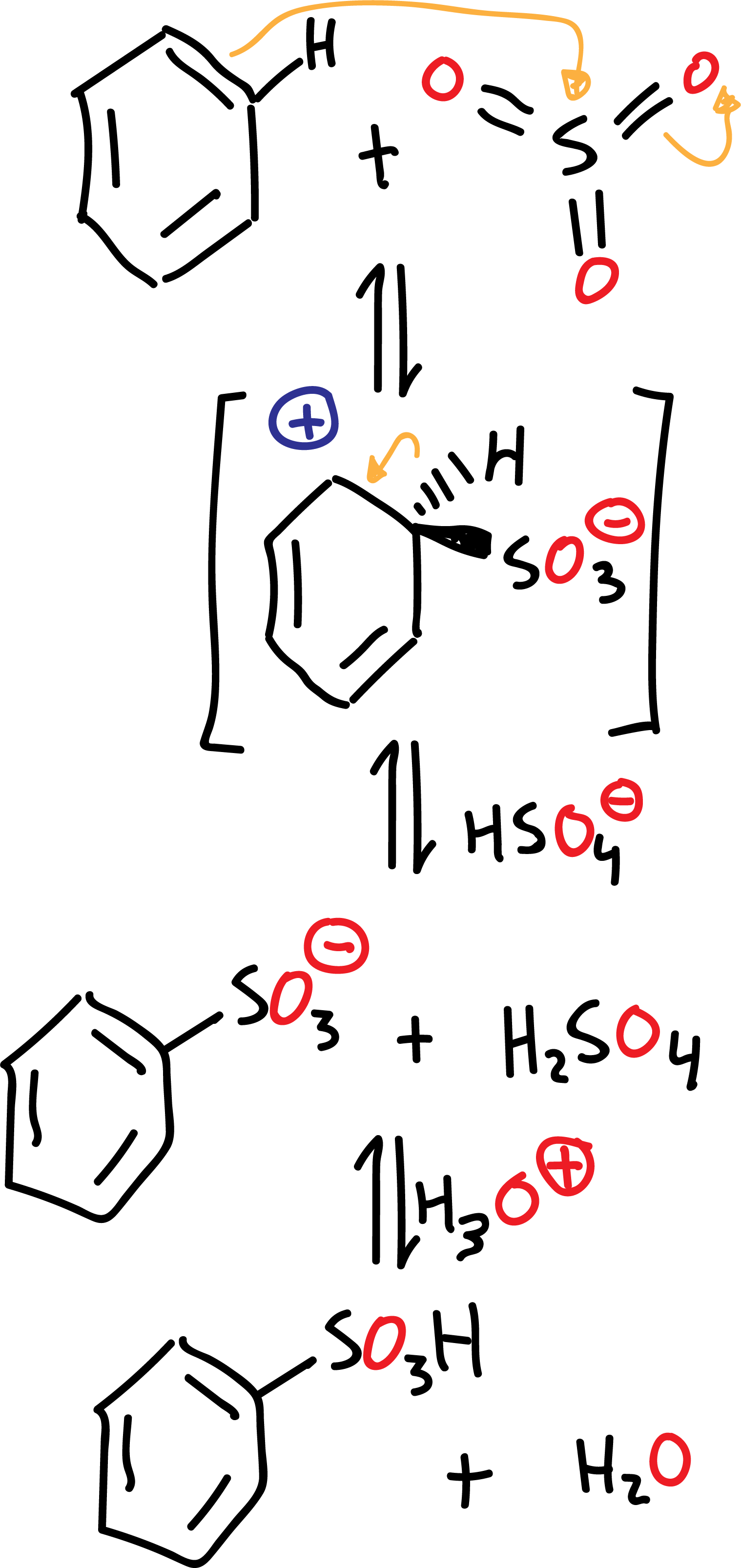
The sulfonation reaction differs from other electrophilic aromatic substitution reactions in that it is an equilibrium process and therefore reversible. The formation of the sulfonation product is favored by eliminating water from the reaction.
Friedel-Crafts alkylation
The alkylation reaction, also known as Friedel-Crafts alkylation, consists of the reaction of an alkyl halide (chloride or bromide) with an aromatic compound in the presence of a Lewis acid of the AlX3 type. Vinyl or aryl halides do not react under these conditions.
The electrophile formed is a carbocation produced by the action of the catalyst.
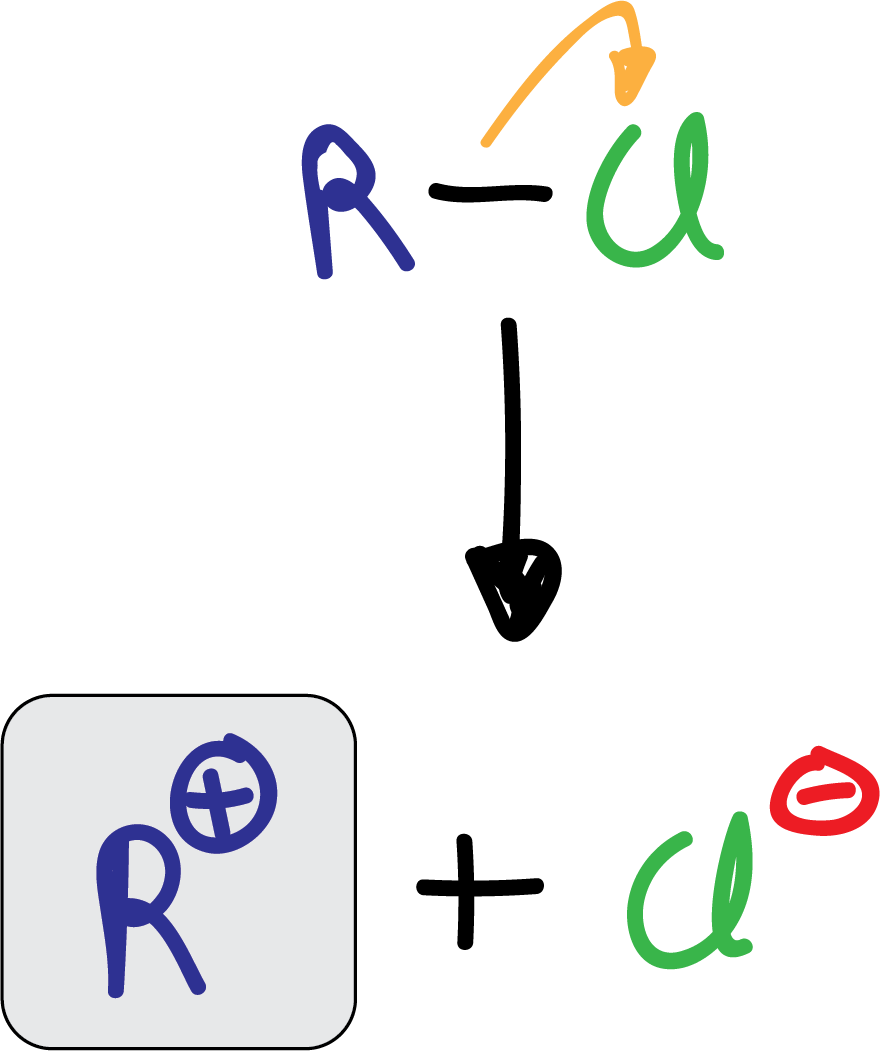
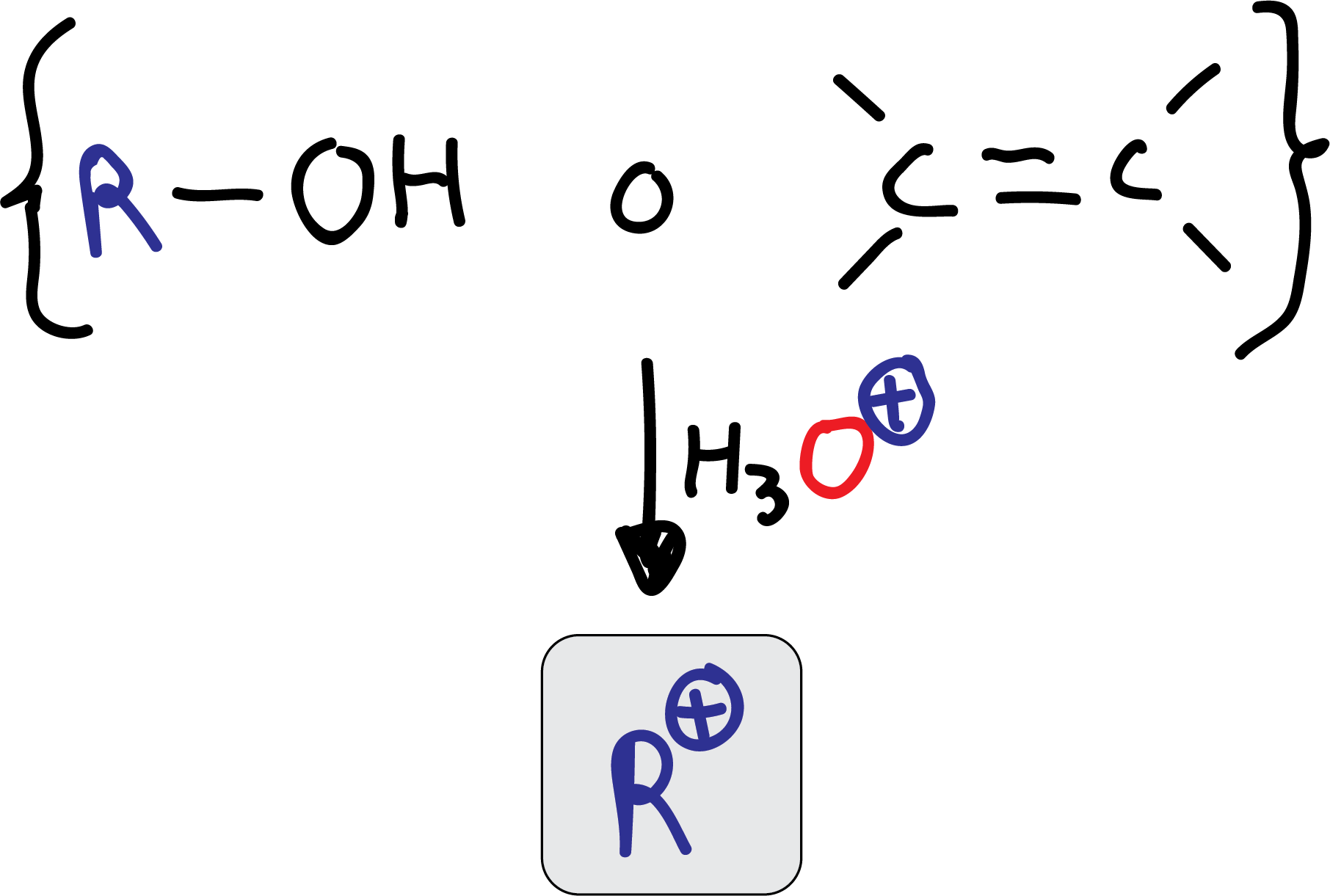
As an alternative to using an alkyl halide to generate the electrophile, alcohols or alkenes, which in the presence of acid, also form carbocations, can be used.
The reaction has a number of limitations.
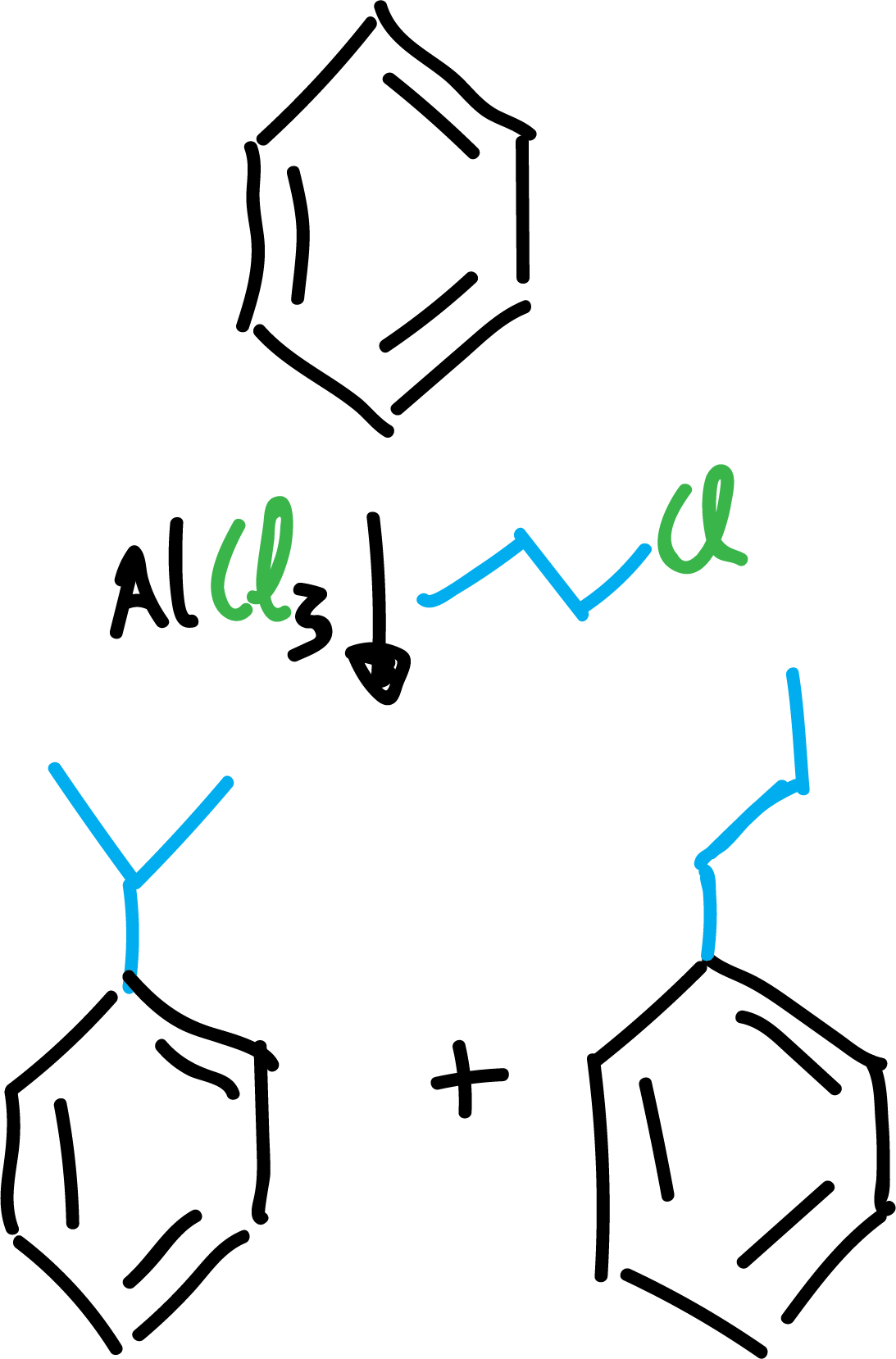
On the one hand, when carbocations are generated, they usually undergo regrouping reactions, obtaining products different from those that should be expected in principle.
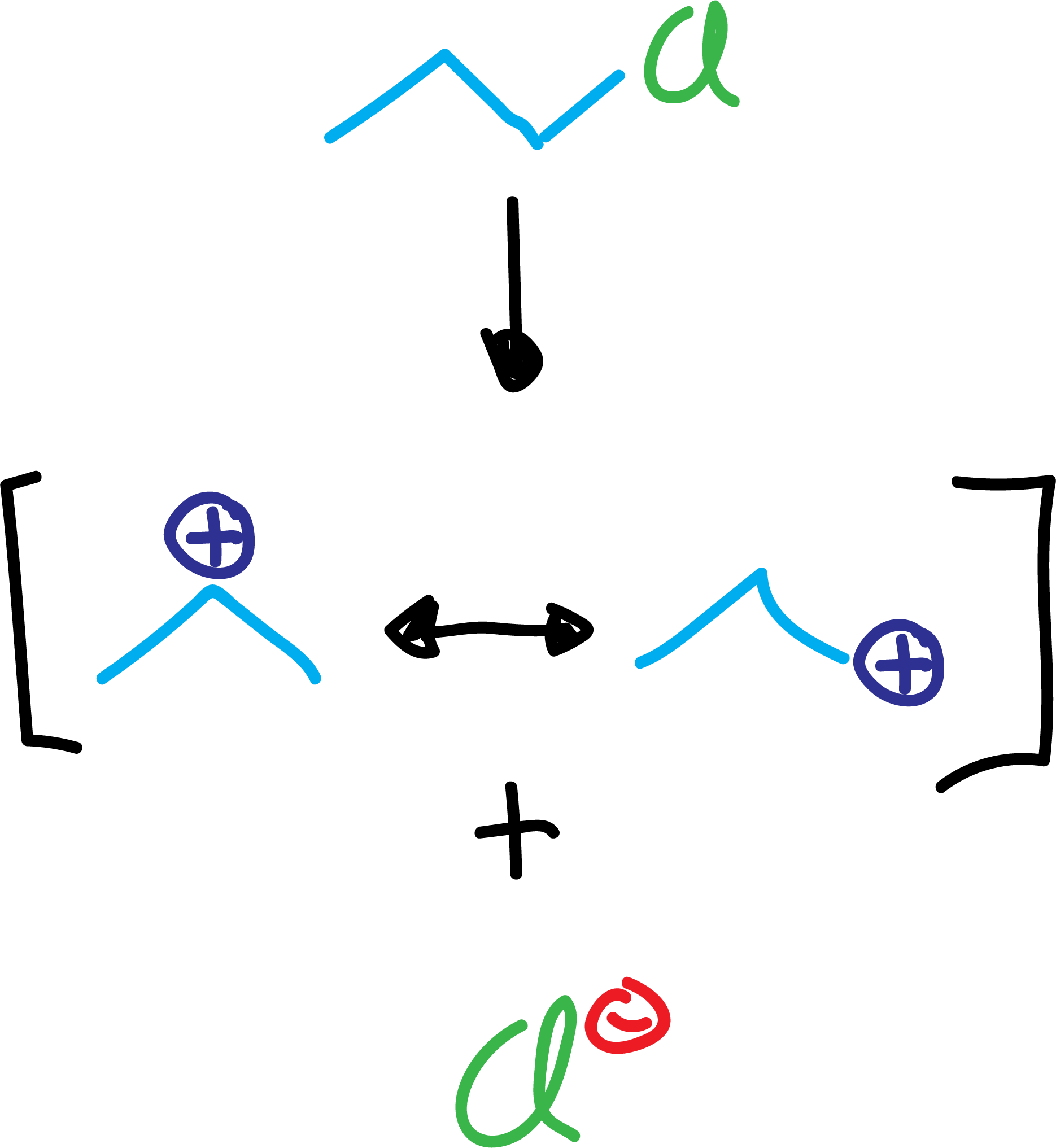
On the other hand, the introduction of alkyl groups turns aromatic rings into molecules more reactive than benzene itself, making it difficult to isolate the monosubstitution product, often resulting in polysubstituted derivatives.
Friedel-Crafts acylation
In Friedel-Crafts acylation, an aromatic ketone is obtained by reaction of the aromatic ring with an acyl chloride in the presence of a Lewis acid, usually AlCl3.
The electrophile that is generated is an acyl cation (R-CO⊕), relatively stable, because it is resonance stabilized.
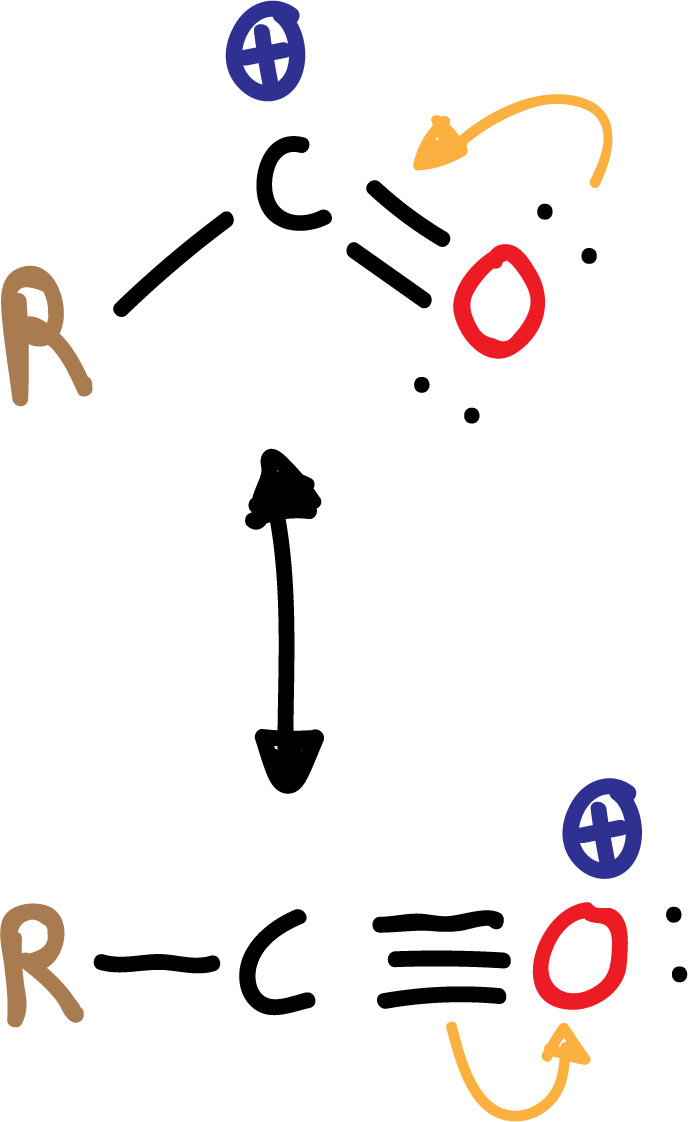
In the acylation reaction no rearrangements occur, as in the case of alkylation.
Electrophilic aromatic substitution (SEAr) on substituted benzenes
Monosubstituted benzenes
When an electrophilic aromatic substitution reaction (SEAr) is carried out on a benzene ring with one or more ring positions with substituents other than hydrogen, two factors are involved in the reaction:
- That the group(s) attached to the aromatic ring increase(s) or decrease(s) the reaction rate with respect to benzene.
- The entry position of the new substituent.
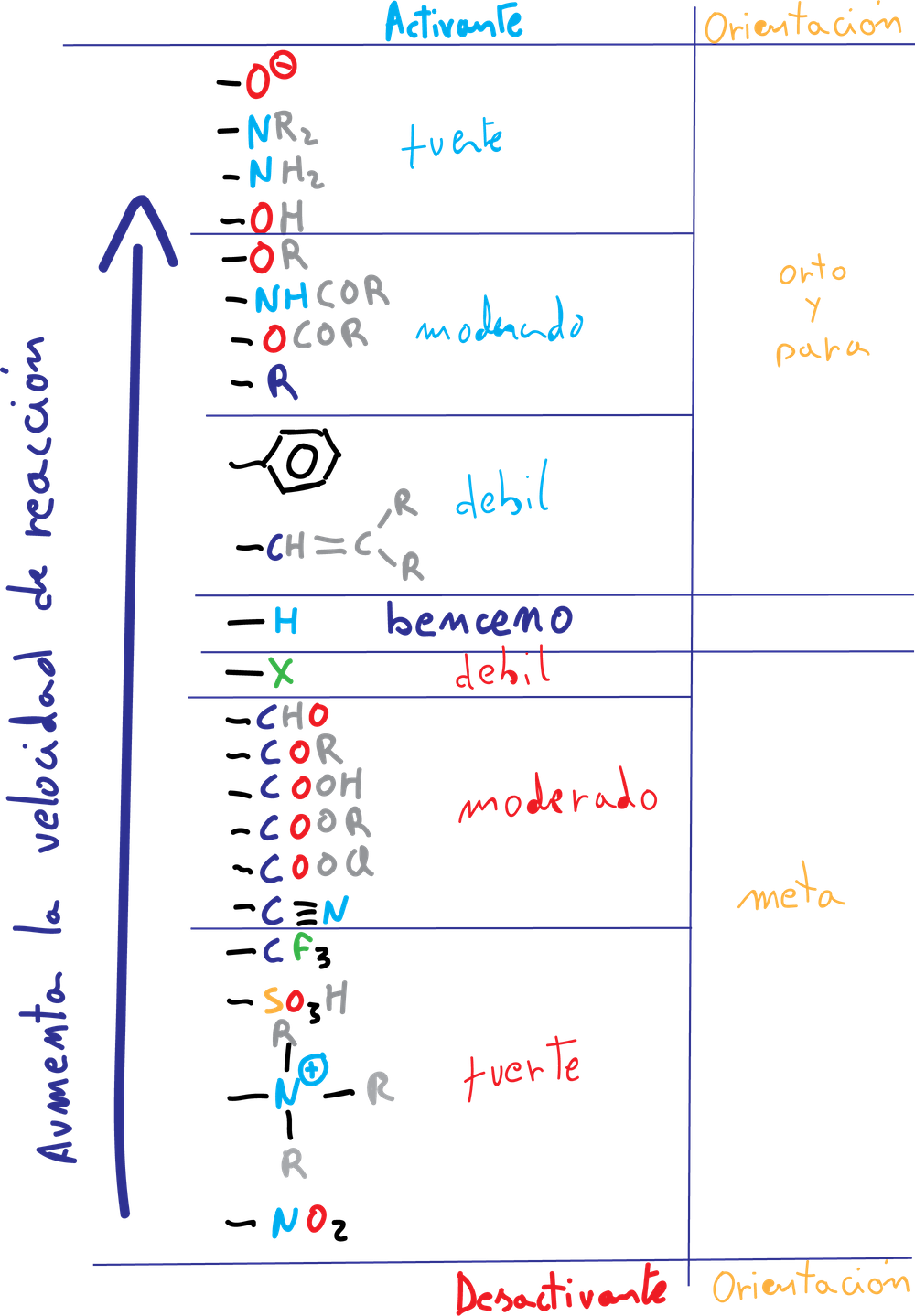
Groups that increase the rate of electrophilic aromatic substitution reaction with respect to benzene are called activating, and those that decrease it are called deactivating. When an electrophile is introduced on a monosubstituted benzene, three isomers can occur:
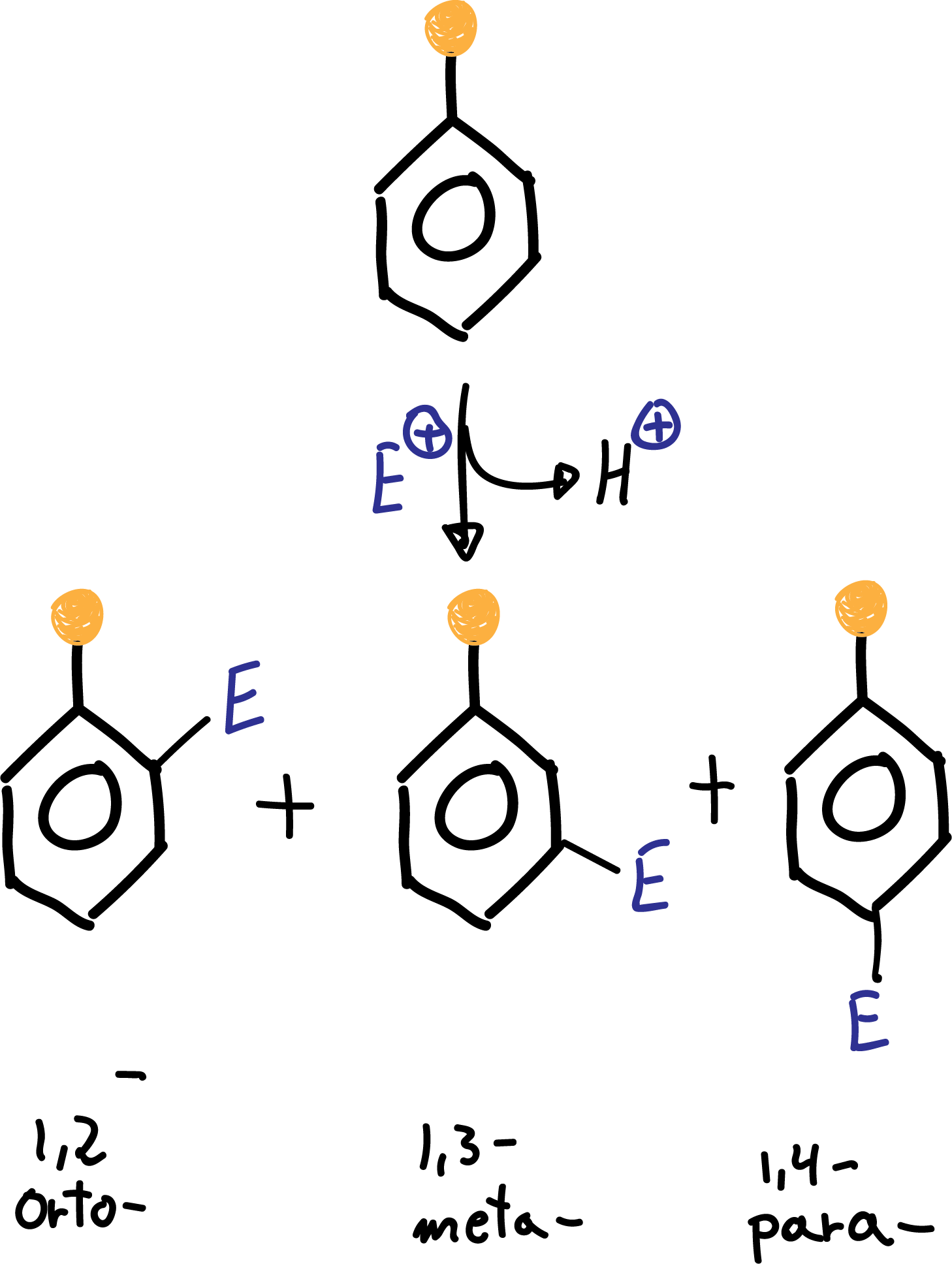
The formation of the major product of the substitution reaction in monosubstituted benzene derivatives can be predicted according to the following classification:
A.- The activating groups A (-NH2, –OR, etc) and halogens produce an increase of the electronic density in the ortho–and para– positions, due to the influence of the non-bonding electron pairs that this type of substituents possess.
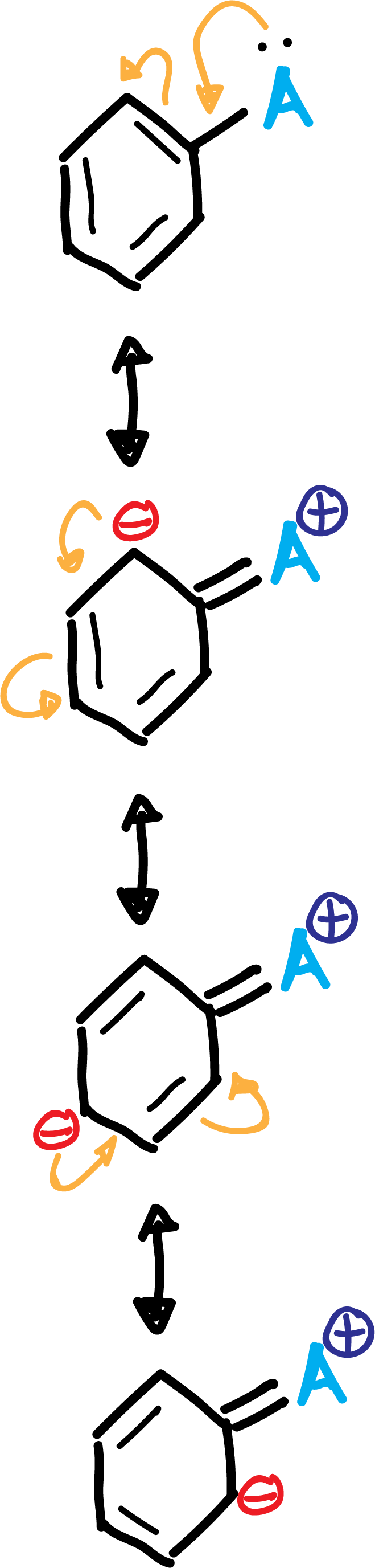
In summary, the activating group A is left with a positive partial charge, while in the ring, in ortho- and para- positions, negative partial charges appear, as shown in the scheme:
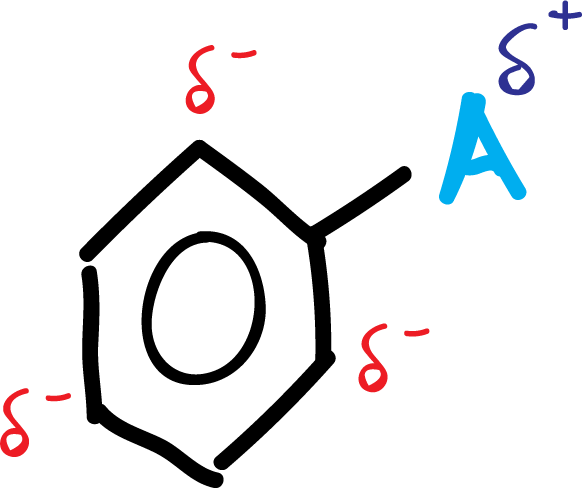
B.- The deactivating groups B (-NO2, -CN, –COO-, etc), except for the halogens, are meta-directing in a new substitution reaction, since they produce an increase of the positive charge density on the ortho- and para-positions, so that the meta-positions are not so disadvantaged.
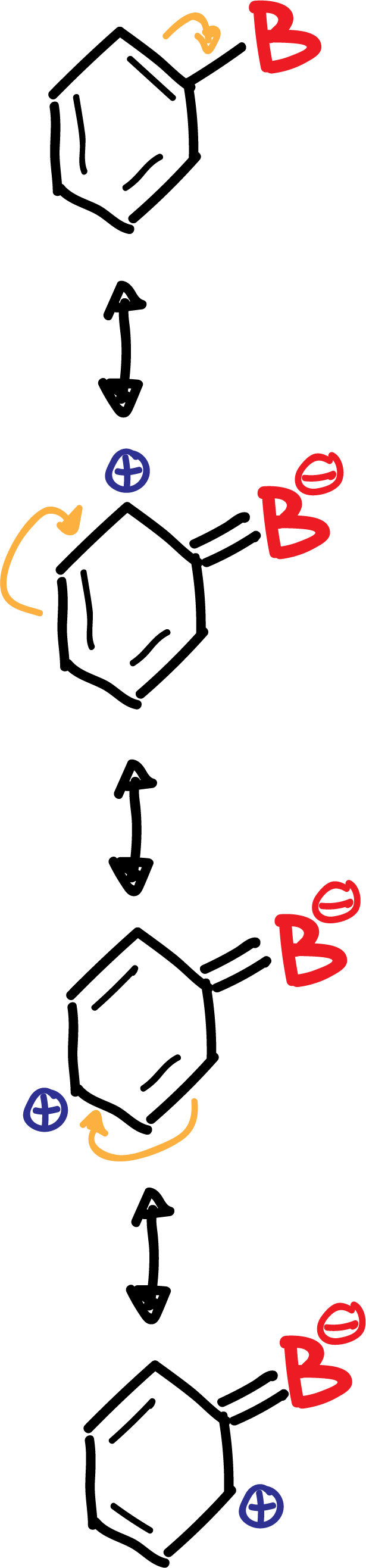
In summary, the deactivating group B is left with a negative partial charge, while in the ring, in ortho- para para-positions, positive partial charges appear, as shown in the scheme:
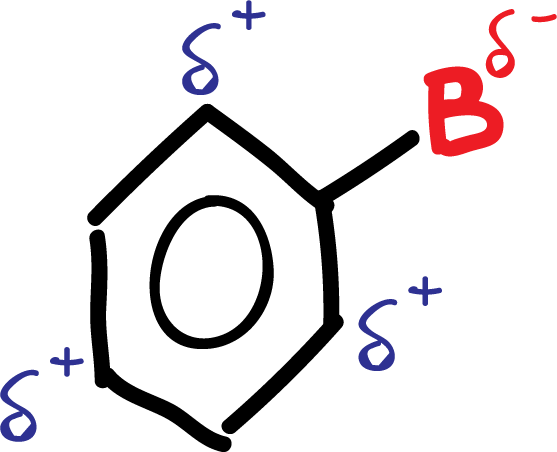
These electron density variations in the aromatic ring of benzene derivatives can be predicted based on the resonance structures of compounds that contribute or withdraw electrons to the π-system of the aromatic ring.
Disubstituted benzenes
When more than one substituent is present, the activation of the benzene ring and the orientation in the attack of the new electrophile can be predicted fairly accurately since the outcome of a new electrophilic aromatic substitution reaction is due to the sum of the individual effects of the groups attached to the aromatic ring.
To predict the orientation of the electrophile attack, two cases are considered:
- Groups with cooperative effect: The attack of an electrophile in one or more positions of the ring is favored.
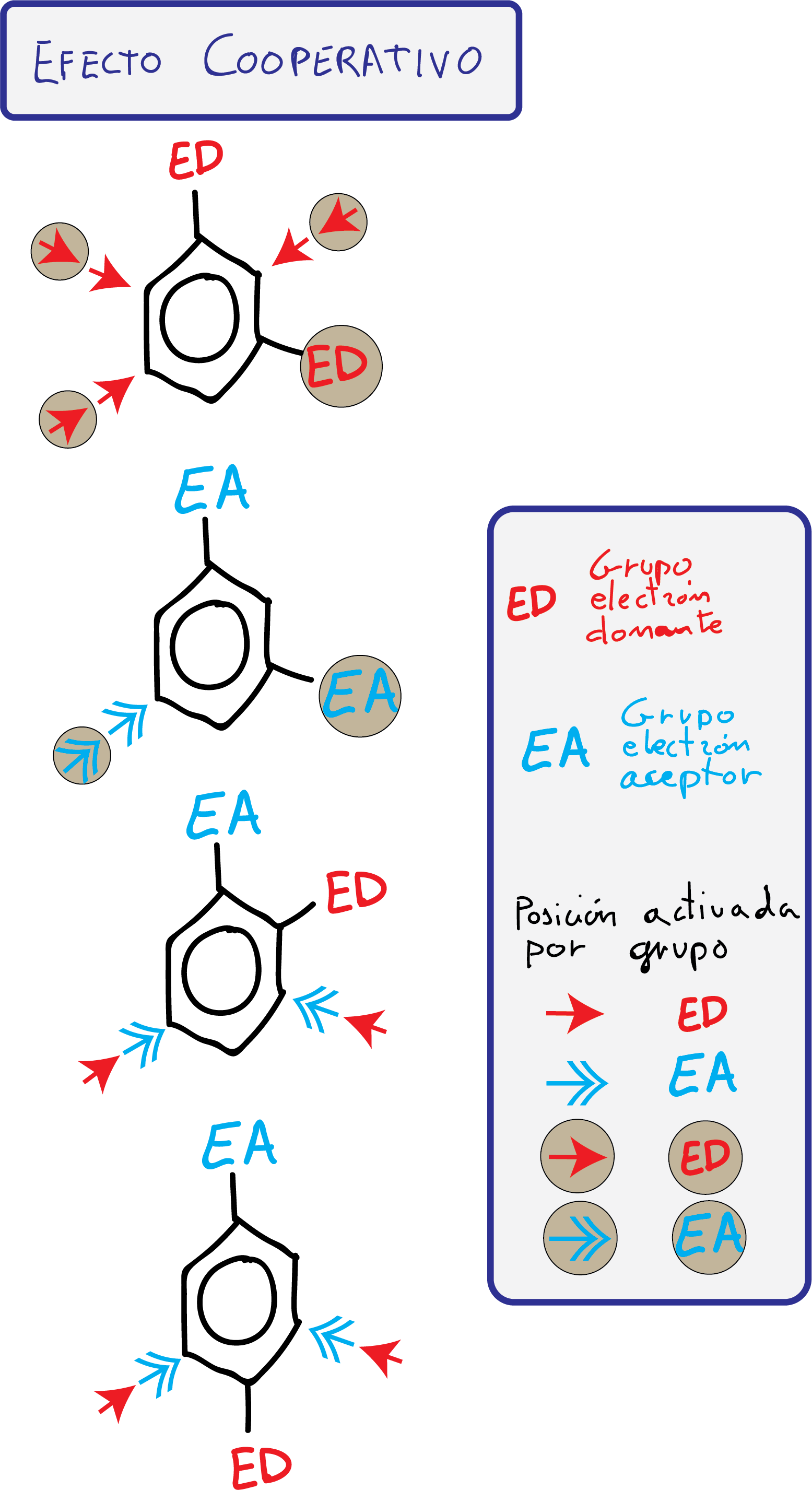
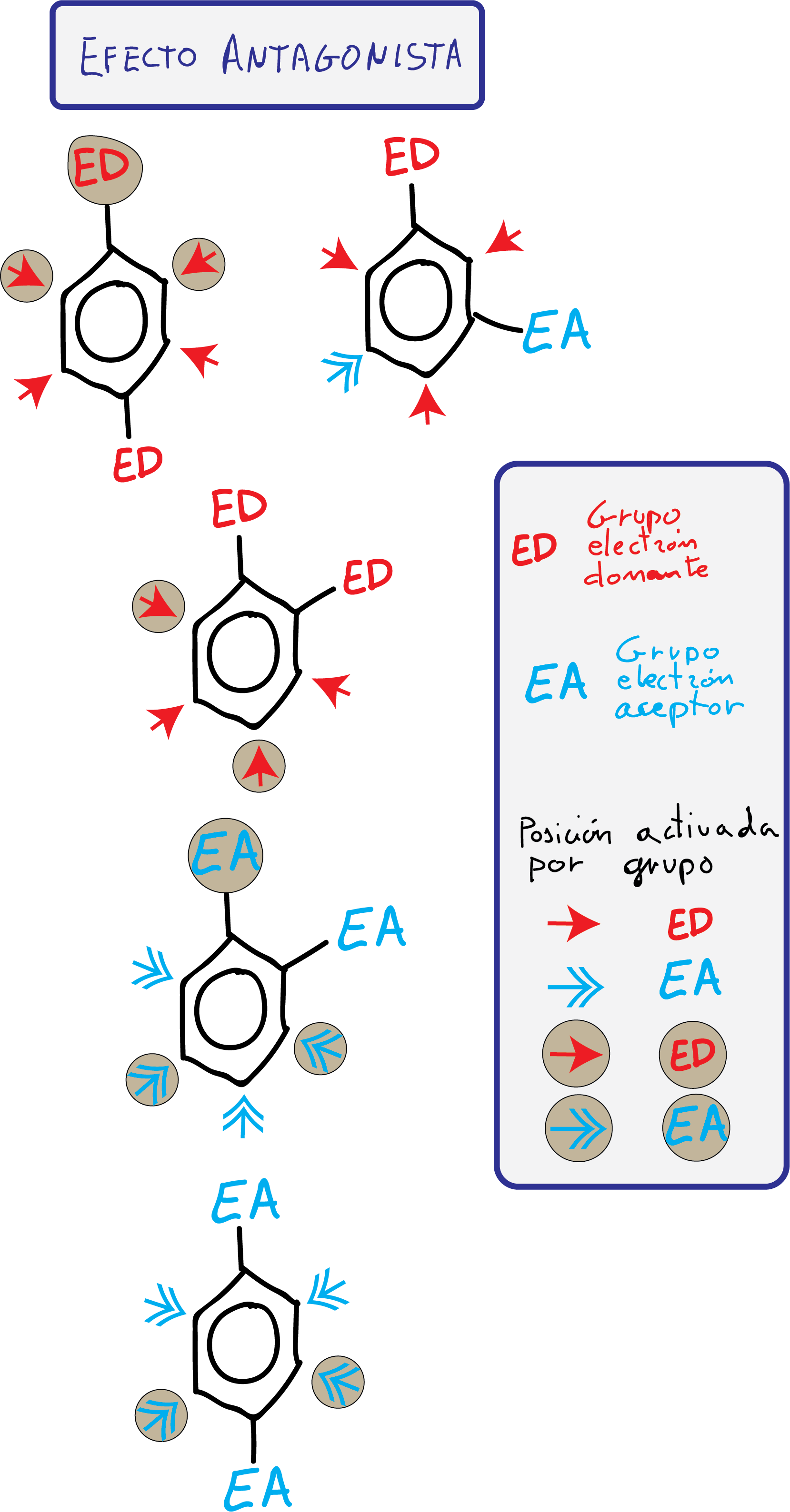
- Groups with antagonistic effect: The substituents present opposing effects or, being effects of the same type, there are no positions that present a differentiated reactivity.
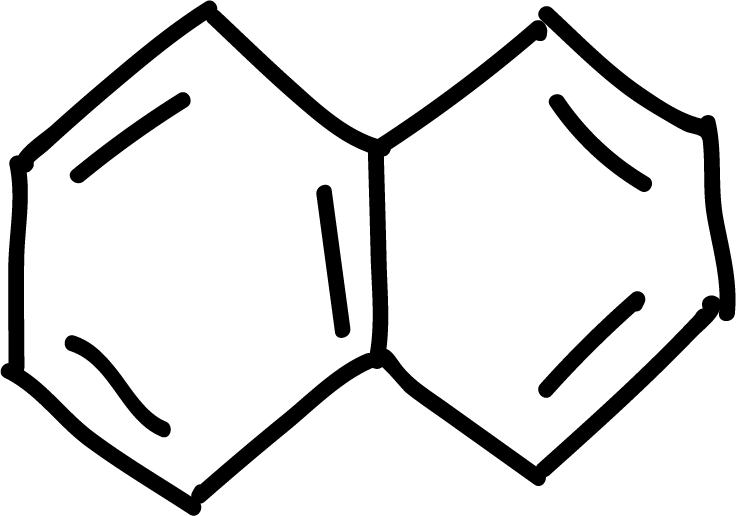
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons such as naphthalene, anthracene and phenanthrene can give the electrophilic aromatic substitution reactions of halogenation, sulfonation or acylation type. Position 1 of naphthalene is the most reactive, while in anthracene and phenanthrene position 9 is the most reactive.
Under normal conditions, this type of product is obtained. However, with sulfonation, being a reversible reaction, it evolves towards the thermodynamically more stable product, which in naphthalene is the one substituted in position 2.
The higher reactivity of these positions is justified by the resonance structures of the transition states.
Disubstituted products and mixtures of products are often obtained, so a detailed study is required for each case.
Nucleophilic aromatic substitution (SNAr)
In aromatic rings with deactivating groups, it is possible to perform the nucleophilic halogen substitution reaction.
For the reaction to be viable it is necessary that in the ortho- or para- positions with respect to the halogen there is a deactivating group such as the -NO2 group (if it is in both simultaneously, the better), and that the reagent used is a good nucleophile.
This type of reaction can occur by means of two mechanisms:
a) Addition-elimination
The presence of an electron-attracting group in the orto– or para–-position is essential for the stabilization of the negative charge of the anion. Without these groups the reaction is not possible.
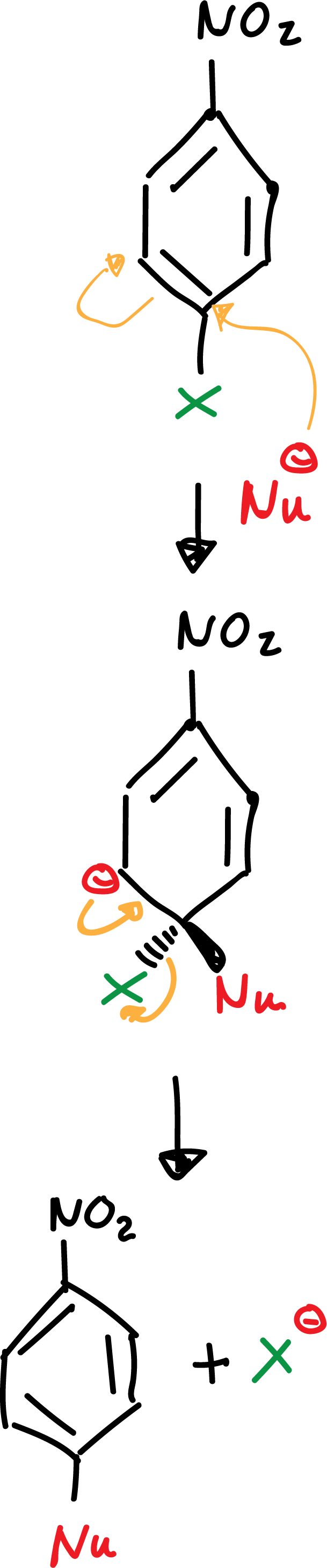
The -NO2 group favors the delocalization of the negative charge on the intermediates formed in the reaction as indicated:
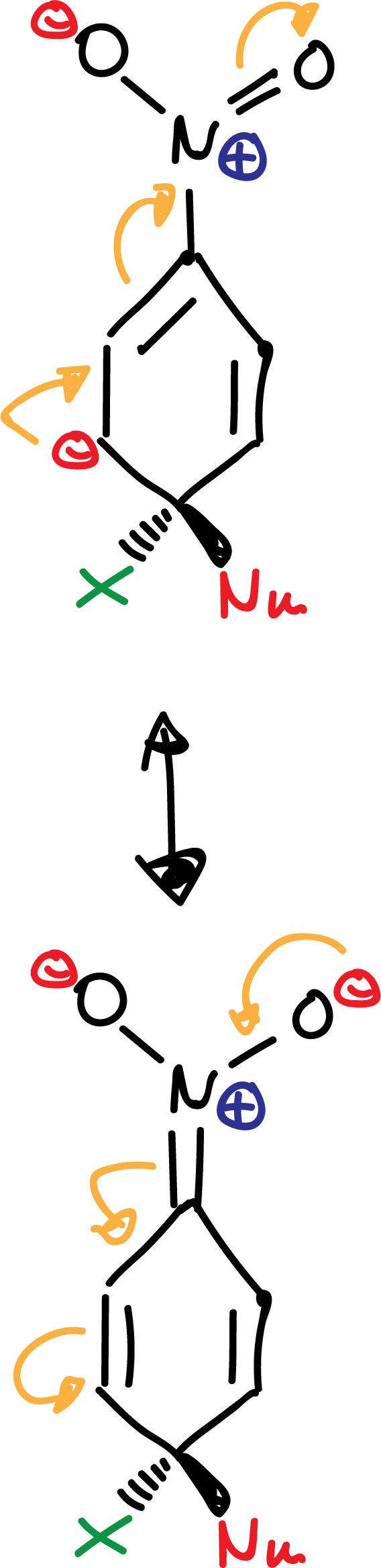
The order of reactivity for halogens is F > Cl > Br > I, just the opposite of that in SN2 reactions.
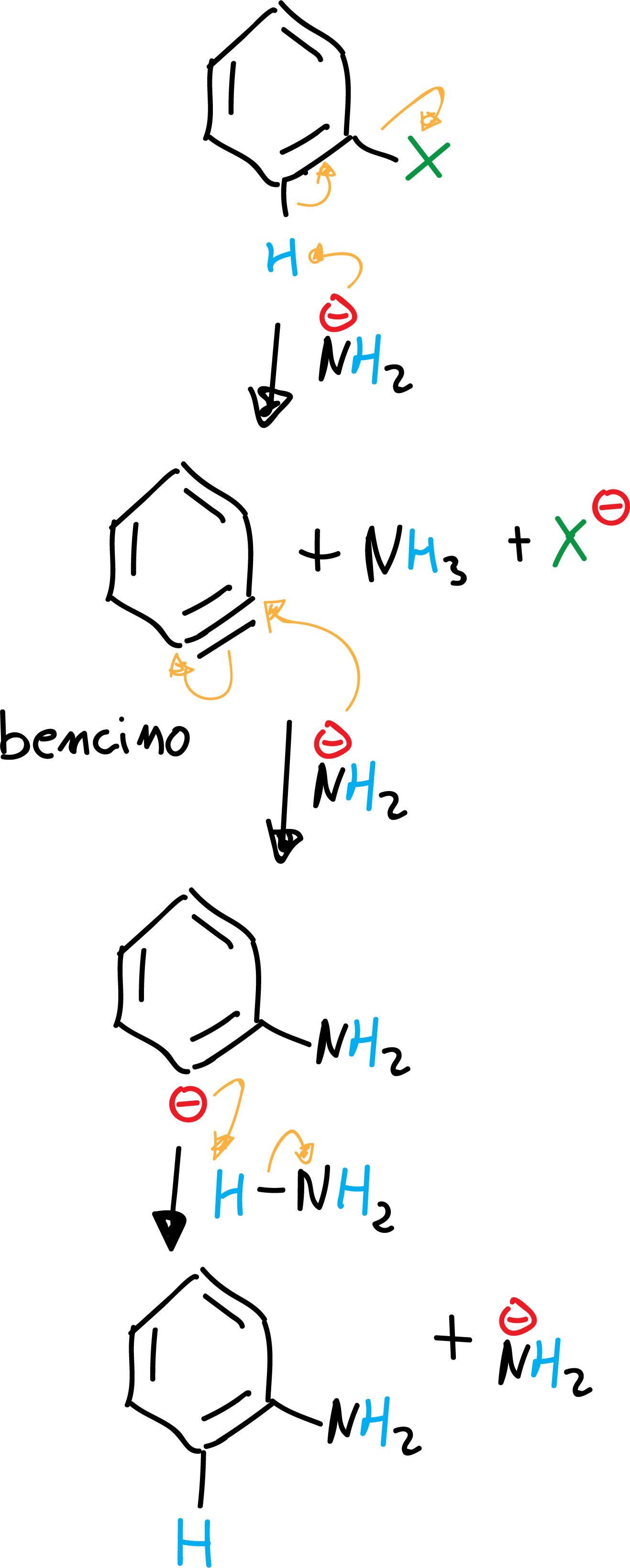
b) Elimination-addition (by means of the benzyne intermediate)
Very strong bases such as sodium or potassium amidide are capable of reacting with aryl halides to give the halogen substitution product for the base. Moreover, this substitution product obtained is itself a nucleophile. The reaction proceeds by an elimination-addition mechanism via an intermediate which is called benzyne.
Additions to the aromatic ring
Catalytic hydrogenation
Despite the stability of benzene, under certain reaction conditions it can undergo addition reactions at high pressure and in the presence of Pt, Pd or Ni.
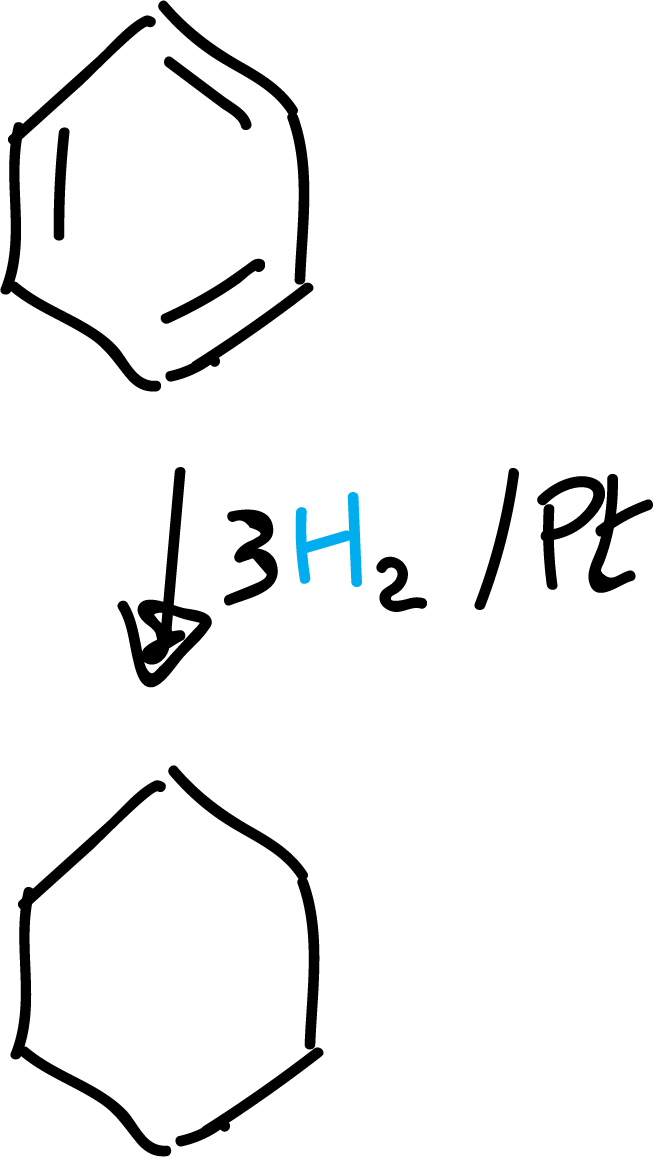
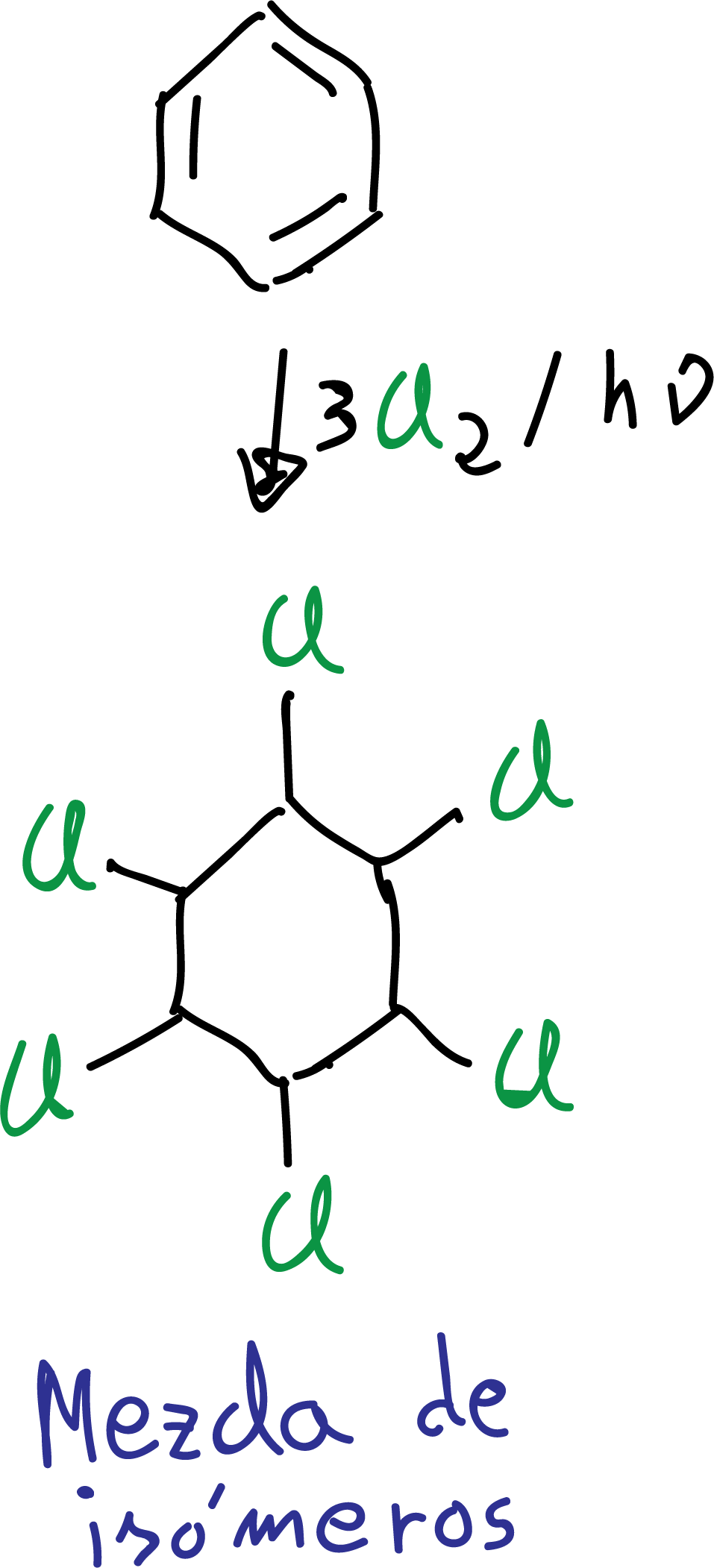
Chlorine addition
Benzene treated with chlorine in the presence of ultraviolet light leads to the formation of 1,2,3,4,5,6-hexachlorocyclohexane as a mixture of stereoisomers.
Birch reduction
It allows the preparation of 1,4-cyclohexadienes from arenes. The reducing agent is sodium, which transfers an electron to the π system of the aromatic ring.
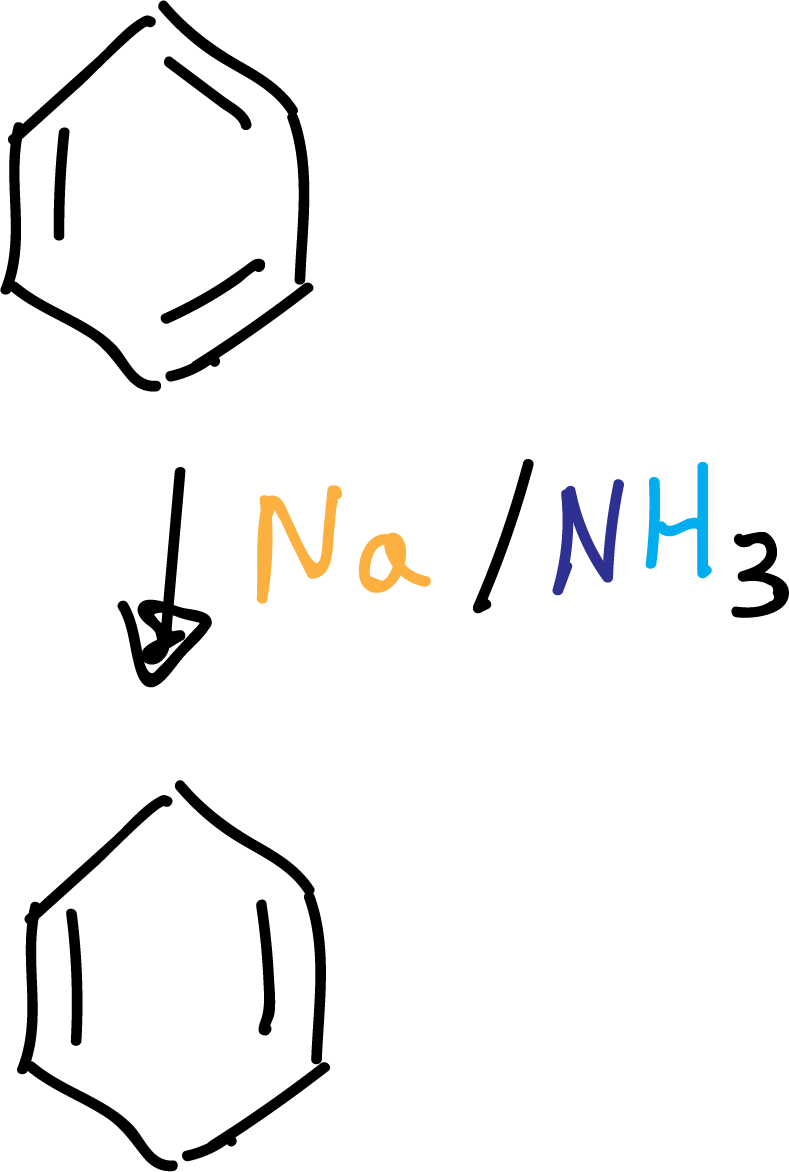
In arenes with substituents exhibiting +I effect, more substituted alkenes are obtained.