Written by J.A Dobado | Last Updated on April 22, 2024
What is Auwers-Skita rule?
Auwers-Skita rule, also called conformational rule, is an empirical rule related to the physical properties of geometrical isomers and their configurations. This rule was initially proposed, in 1920, simultaneously by Karl von Auwers and by A. Skita, and was restated by Norman Allinger in 1956. This rule was extensively applied during the assignment of the structures of isomers before the NMR techniques were commonly available.
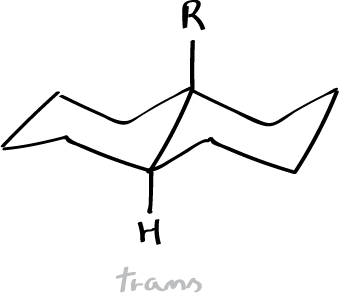
- R = OH 54 ºC
- R = NH2 -25 ºC
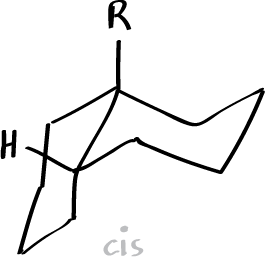
R = OH 65 ºC
R = NH2 -13 ºC
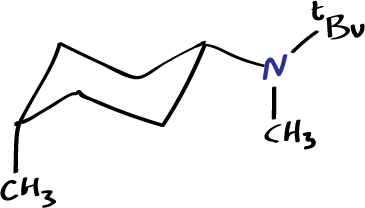
cis–N-methyl-N-neopentyl-4-methylcyclohexylamine, nD20 = 1.457, b.p. 90–91 ºC (4.5 mmHg).
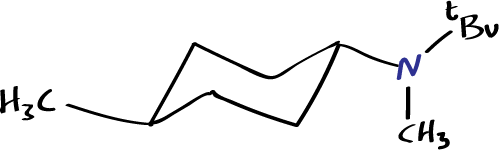
trans–N-methyl-N-neopentyl-4-methylcyclohexylamine, nD20 = 1.452, b.p. 88–89 ºC (4.5 mmHg).
The Auwers-Skita rule, applying to alicyclic epimers not differing in dipole moment, states that the isomer of smaller molecular volume and therefore higher physical constants (e.g., density, index of refraction, and boiling point) has the higher heat content (enthalpy), however, molecules of higher enthalpy have a lower melting point. Between the pair of cis– and trans– isomers, cis-compounds are higher in specific gravity and refractive index but smaller in molecular refraction. Between stereoisomers, the transform of a pair of stereoisomers has the lower density, boiling point, and refractive index.
The Auwers-Skita rule can even be used to assign the conformers, in which the conformer of higher enthalpy has a lower molecular volume. In cyclic stereoisomers, when the substituents are bound to configurationally identical ring systems, the isomer with the higher density and the higher refractive index is that which has the higher heat content. Usually, the (higher) boiling point and (lower) molar refraction can be related in a similar manner. In other words, isomers having the greater number of axial substituents within a series will have the higher boiling point and refractive index and the greater density.
Although the Auwers-Skita rule has been proved successful with a variety of disubstituted cyclohexanes, it is recognized that such an empirical rule is of limited reliability, especially in complex systems, such as the configuration for isopinocampheol, 3-methylcyclohexanol, thiabicyclo[3.3.0]-octanes, and other 1,3-disubstituted cyclohexenes.
References
- Skita, A. (1920), Über die geometrische Isomerie der Polymethylene. [On the geometric isomerism of polymethylenes] Ber. dtsch. Chem. Ges. A/B, 53: 1792-1806. https://doi.org/10.1002/cber.19200530926
- v. Auwers, K. (1920), Über Beziehungen zwischen Konstitution und physikalischen Eigenschaften hydroaromatischer Verbindungen. [On relationships between constitution and physical properties of hydroaromatic compounds.] Justus Liebigs Ann. Chem., 420: 84-111. https://doi.org/10.1002/jlac.19204200104
- Skita, A. (1922), Über Konfigurationsbestimmungen bei stereoisomeren Hexamethylenen. [On configurational determinations in stereoisomeric hexamethylenes.] Justus Liebigs Ann. Chem., 427: 255-280. https://doi.org/10.1002/jlac.19224270205
- Skita, A. and Schneck, A. (1922), Über die Stereoisomerie der cyclischen Kohlenwasserstoffe. [On the stereoisomerism of cyclic hydrocarbons] Ber. dtsch. Chem. Ges. A/B, 55: 144-152. https://doi.org/10.1002/cber.19220550119
- Skita, A. (1923), Über die Stereochemie der Hexahydro-toluidine. [On the stereochemistry of hexahydro-toluidines.] Ber. dtsch. Chem. Ges. A/B, 56: 1014-1023. https://doi.org/10.1002/cber.19230560505
- Skita, A. (1923), Zur Stereochemie der trisubstituierten Cyclohexane. [On the stereochemistry of trisubstituted cyclohexanes.] Ber. dtsch. Chem. Ges. A/B, 56: 2234-2244. https://doi.org/10.1002/cber.19230561006
- Skita, A. (1923), Über die Stereochemie cyclischer Alkohole, Aldehyde und Carbonsäuren. [On the Stereochemistry of Cyclic Alcohols, Aldehydes and Carboxylic Acids] Justus Liebigs Ann. Chem., 431: 1-30. https://doi.org/10.1002/jlac.19234310102
- Notes – The Relative Stabilities of Cis and Trans Isomers. II. The Decalin and Hydrindan Ring Systems.
N. Allinger
The Journal of Organic Chemistry 1956 21 (8), 915-917
DOI: 10.1021/jo01114a604 - The Relative Stabilities of cis and trans Isomers. III. The Cyclodecenes
Norman L. Allinger
Journal of the American Chemical Society 1957 79 (13), 3443-3446
DOI: 10.1021/ja01570a034 - On the Molecular Geometry of trans-Cycloöctene
Norman L. Allinger
Journal of the American Chemical Society 1958 80 (8), 1953-1955
DOI: 10.1021/ja01541a044 - Notes. The Relative Stabilities of cis and trans Isomers. X. The 1,3-Cyclohexanedicarboxylate Esters.
Norman Allinger and Ronald Curby, Jr.
The Journal of Organic Chemistry 1961 26 (3), 933-935
DOI: 10.1021/jo01062a600 - van Bekkum, H., van Veen, A., Verkade, P.E. and Wepster, B.M. (1961), On the so-called Auwers-Skita Rule, The conformational rule, and the isomer sequence rule. Recl. Trav. Chim. Pays-Bas, 80: 1310-1322. https://doi.org/10.1002/recl.19610801204
- Feltkamp, H. and Thomas, K.D. (1965), Anwendung der Auwers-Skita-Regel auf stereoisomere Amine. [Application of the Auwers-Skita rule to stereoisomeric amines.] Justus Liebigs Ann. Chem., 685: 148-154. https://doi.org/10.1002/jlac.19656850118
- Conformational Studies in the Cyclohexane Series. 1. Experimental and Computational Investigation of Methyl, Ethyl, Isopropyl, and tert-Butylcyclohexanes
Kenneth B. Wiberg, Jack D. Hammer, Henry Castejon, William F. Bailey, Eric L. DeLeon, and Ronald M. Jarret
The Journal of Organic Chemistry 1999 64 (6), 2085-2095
DOI: 10.1021/jo990056f