Written by J.A Dobado | Last Updated on April 22, 2024
What are ketones?
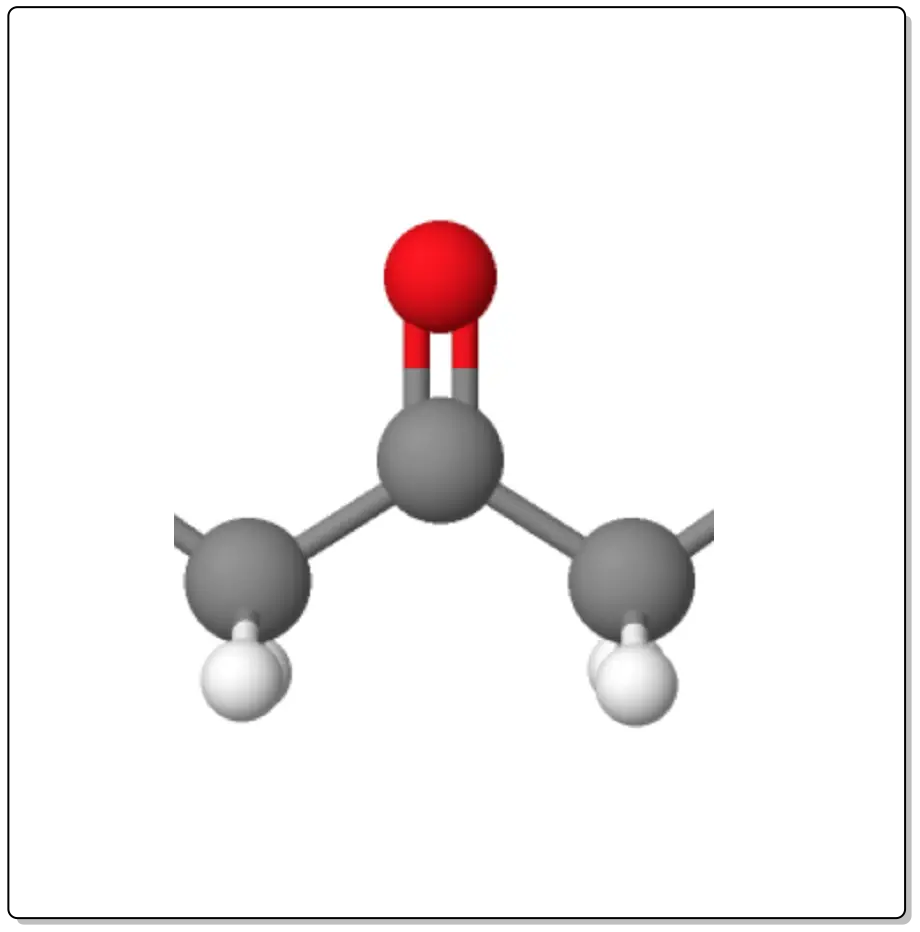
Ketones are organic compounds with the functional group >C=O (carbonyl group), with a general formula RC(=O)R’, where -R and -R’ can be different carbon substituents. The simplest is formaldehyde, with R and R’ = methyl and formula H3CC(O)CH3 (called ketone).
Applications
They are produced on a large scale in industry, e.g. as solvents, pharmaceuticals, or polymer precursors. Worldwide, the ketones produced on the largest scale are: acetone, methyl ethyl ketone and cyclohexanone.
Nomenclature
Follow the link for a summary of the formulation and nomenclature rules for ketones.
Reactions of ketones
Ketones and aldehydes have common reactivity similarities due to the presence of the carbonyl group, but they also have distinguishing characteristics. Therefore, ketone reactions are discussed together with aldehyde reactions.
Analysis of ketones
Ketones and aldehydes have characteristic reactions in common due to the presence of the carbonyl group. However, they also have different reactions due to the property of aldehydes to oxidize to acids by the action of mild oxidants. Therefore, the analysis of ketones is approached together with that of aldehydes.