Written by J.A Dobado | Last Updated on April 22, 2024
What are carboxylic acids?
Carboxylic acids are compounds with the functional group -COOH (carboxyl group). Depending on the substituent to which it is attached, they are classified as aromatic or aliphatic.
If they have more than one carboxyl group, they are called dicarboxylic acids (with two) or polycarboxylic acids (more than two).
Acid-base properties
Carboxylic acids dissociate to give a carboxylate ion RCOO⊖ and a proton H⊕, with pKa values on the order of 5 (e.g. MeCOOH pKa = 4.7). The pKa or pKb values of the conjugate acid or base are obtained from the following relationship: pKa + pKb = 14.
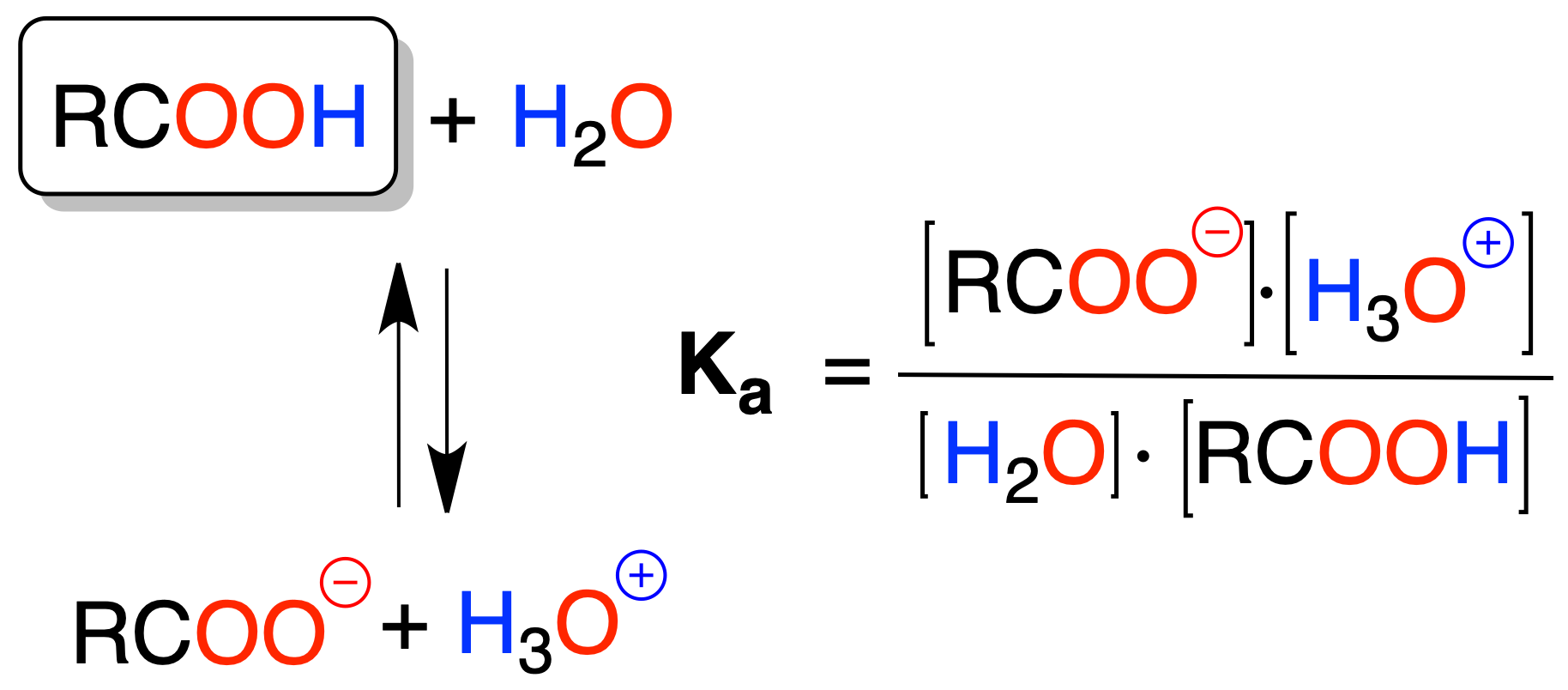
They are not as acidic as inorganic acids, but much more so than other organic compounds; for example, acetic acid is 1011 times more acidic than alcohols.
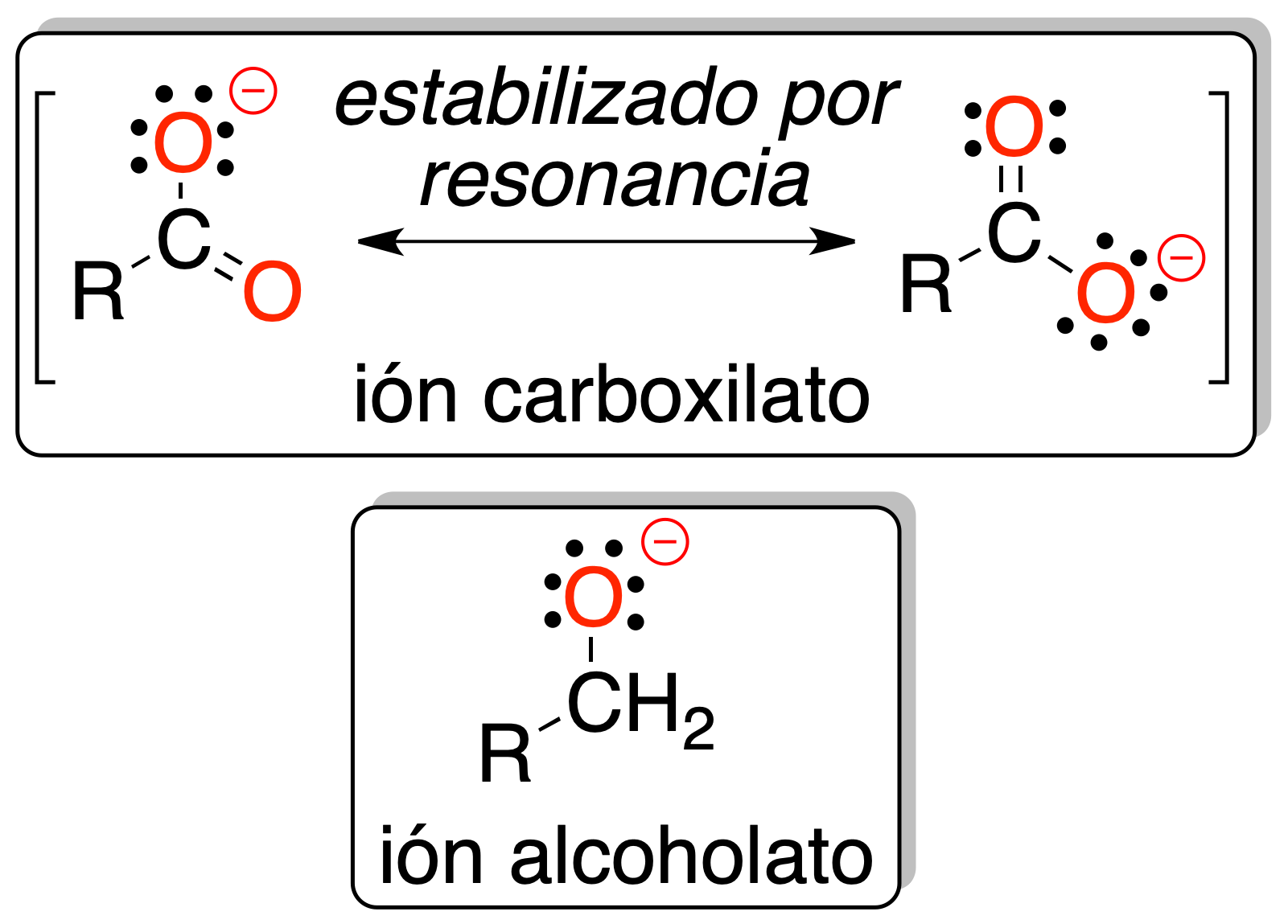
All this is because the negative charge is delocalized on several atoms of the -COO⊖ functional group and therefore makes it more stable than the alkoxide (O⊖) in the case of alcohols.
Therefore, carboxylic acids react with bases to give the corresponding salts and water. In Appendix C, pKa values for the most common carboxylic acids are shown.
Nomenclature of carboxylic acids
Follow the link for a summary of the rules of formulation and nomenclature of carboxylic acids.
Reactions of carboxylic acids
In addition to the acid-base reactions of carboxylic acids, the main organic reactions of these functional groups and their derivatives are described in the section on carboxylic acid reactions.
Analysis of carboxylic acids
To distinguish a carboxylic acid, insoluble in water, from other acidic compounds, it is sufficient to resort to solubility tests, since they are the only ones soluble in a NaOH/NaHCO3 solution (with the exception of some nitrophenols). The water-soluble ones give off CO2 from the bicarbonate solution.
Other compounds that also give CO2 are amine salts, sulfonic acids and those acid derivatives that hydrolyze easily giving acid products.
To distinguish a carboxylic acid, insoluble in water, from other acidic compounds, it is sufficient to resort to solubility tests, since these are the only ones soluble in a NaOH/NaHCO3 solution (with the exception of some nitrophenols).
They show a very broad and characteristic OH band in the IR spectrum, in addition to a carbonyl band near 1700 cm-1.
Carboxylic acids can be characterized by the formation of the following derivatives:
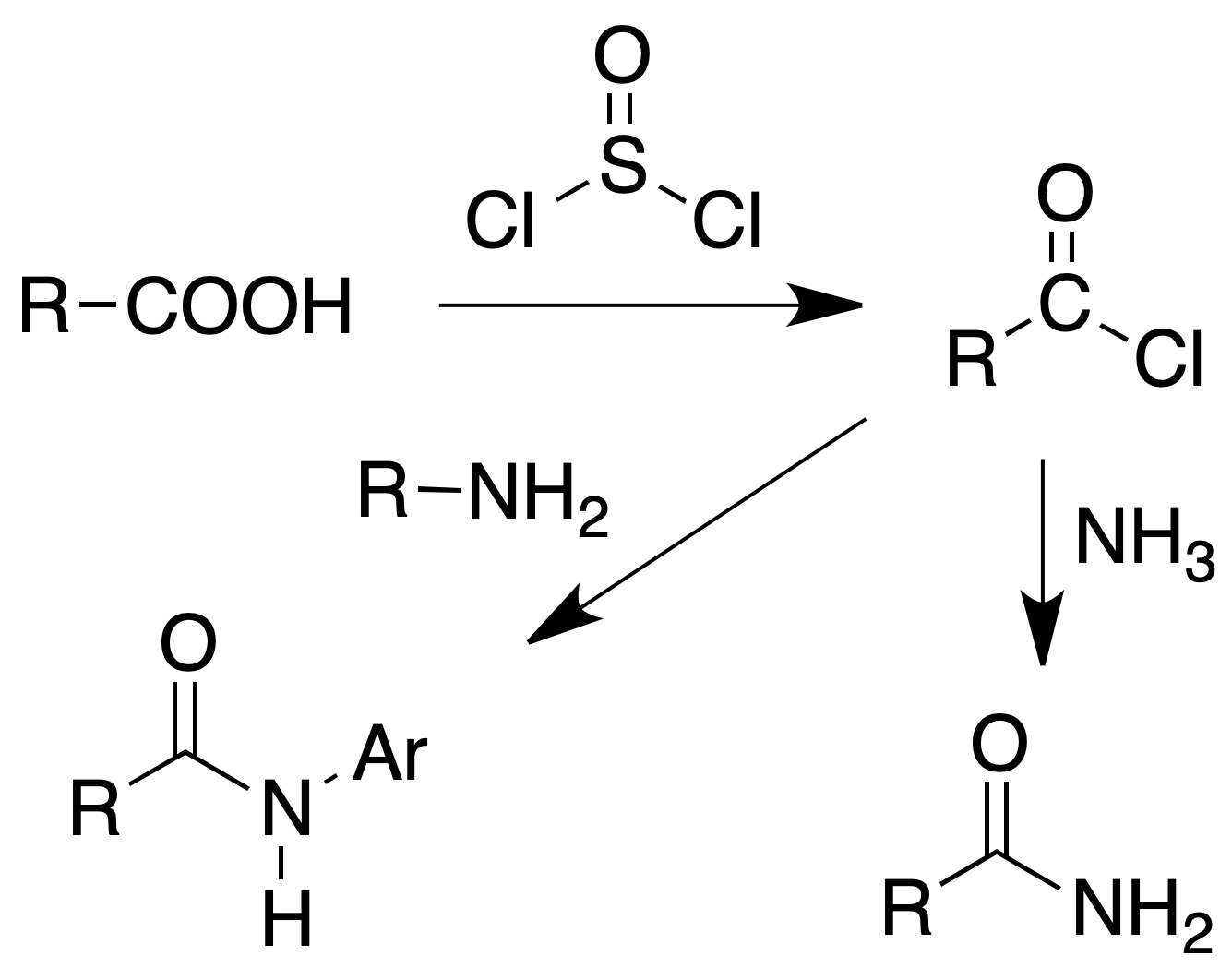
Formation of amides
Procedure : In a 25 ml flask, equipped with reflux and calcium chloride tube, 100 mg of the acid (or its sodium salt) are placed and 4 ml of thionyl chloride are added. The mixture is boiled gently for 30 min and then the mixture is poured over 15 ml of concentrated ammonia cooled with ice. The precipitated amide is collected by filtration.
If instead of the amide it is desired to prepare anilide or p-toluide, the excess of thionyl chloride is distilled off (b.p. = 78 °C). The acid chloride is dissolved in 5 ml of benzene and a solution of 2 g of the amine in 15 ml of benzene is added.
The reaction mixture is shaken with 5 ml of dilute HCl (to remove excess aniline) and decanted. The benzene layer is washed with 5 ml of water, the solvent is removed and the anilide is recrystallized from water or EtOH/H2O.
Phenacyl esters
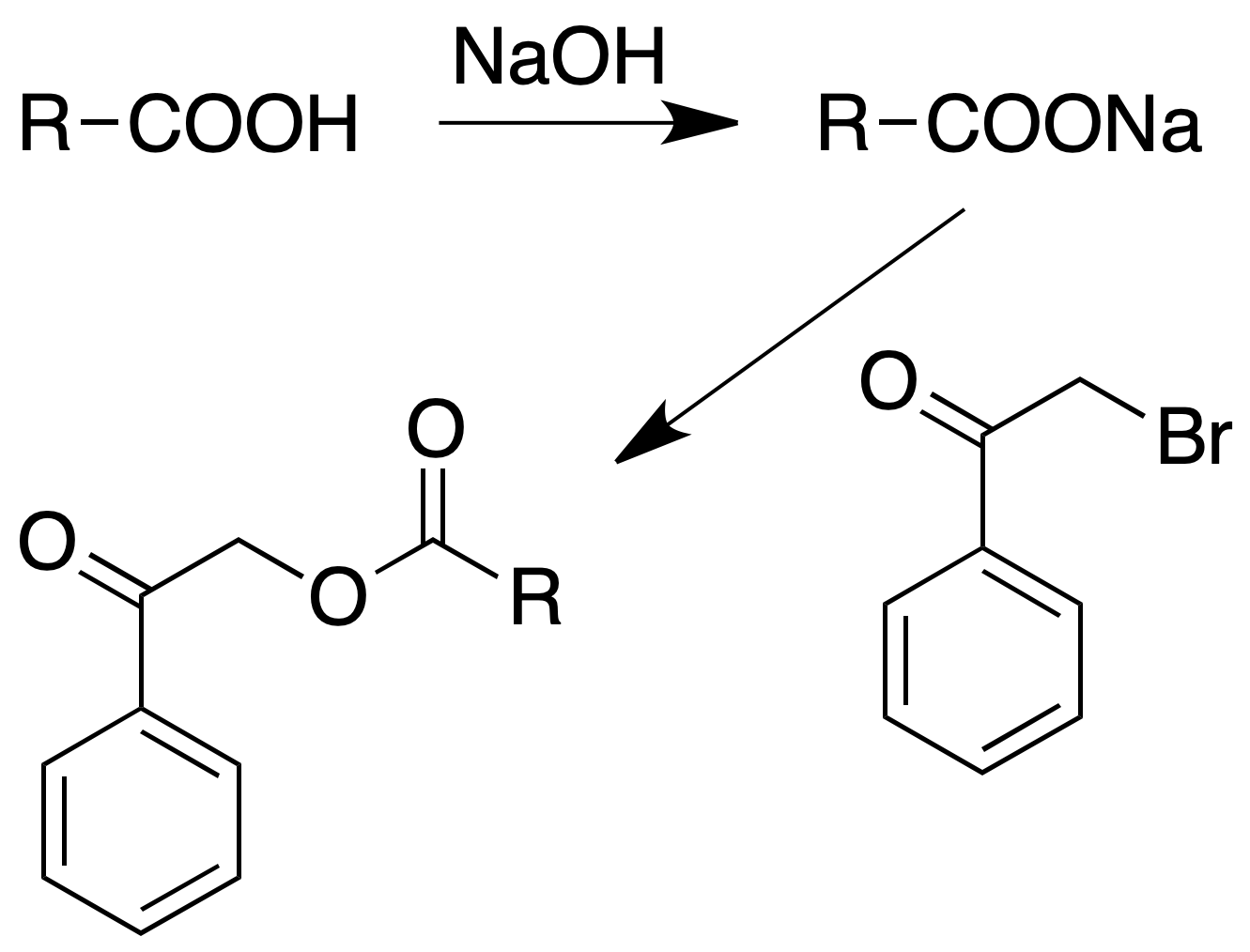
Procedure : Place in a flask provided with reflux 150 mg of acid and 1 ml of water. To add a drop of phenolphthalein and to neutralize with NaOH to 10 % until pink color. To add one or two drops of diluted HCl so that the color disappears.
Add an alcoholic solution of phenacyl bromide (150 mg in about 6 ml of alcohol).
Boil the solution at reflux for at least 2 h; cool, add 1 ml of water and scrape with a stirring rod to induce crystallization.
Filter the solid ester, washing it with 5% sodium carbonate solution and then several times with cold water.
If it were the sodium salt, dissolve about 150 mg of it in water, add a drop of phenolphthalein and adjust the pH as in the previous case.