What is Baeyer-Villiger oxidation?
The Baeyer-Villiger oxidation, also known as the Baeyer-Villiger reaction or Baeyer-Villiger rearrangement, was first reported in 1899 by Baeyer and Villiger.
This chemical reaction involves the oxidation of ketones or cyclic ketones into esters or lactones using peracids (R-CO3H) or hydrogen peroxide H2O2.
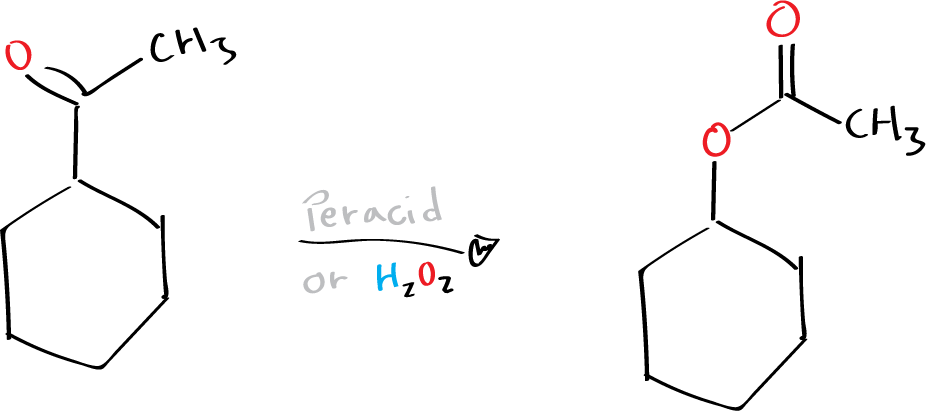
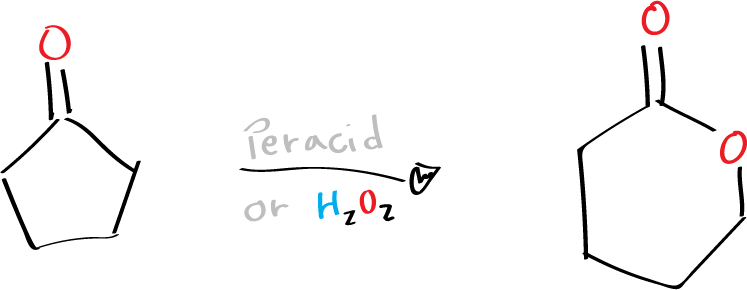
Peroxybenzoic acid, m-chloroperoxybenzoic acid (mCPBA), peroxyacetic acid, and trifluoroperoxyacetic acid are typical peracids employed in this oxidation process (see list of acronyms). In the Baeyer-Villiger oxidation, the more substituted group usually migrates preferentially, and the migratory aptitudes of these groups follow the order of tertiary > secondary > cyclohexyl > benzyl > phenyl > primary > methyl. More significantly, the stereochemistry and chirality of the migrating group are retained during the migration process.
The Baeyer-Villiger oxidation is a useful reaction for the synthesis of a wide range of compounds, including pharmaceuticals, fragrances, and flavorings. It is also used in the production of plastics and polymers..
One of the advantages of the Baeyer-Villiger oxidation is that it allows for the introduction of oxygen into a molecule without the need for expensive and potentially hazardous reagents, such as chromium(VI) compounds. It is also a useful alternative to traditional oxidation reactions, such as the Swern oxidation, which can be limited by the availability of certain reagents or the sensitivity of the starting material..
References
- Baeyer, A. and Villiger, V. (1899), Einwirkung des Caro’schen Reagens auf Ketone. [Action of Caro’s reagent on ketones.] Ber. Dtsch. Chem. Ges., 32: 3625-3633. https://doi.org/10.1002/cber.189903203151
- Baeyer, A. and Villiger, V. (1900), Ueber die Einwirkung des Caro’schen Reagens auf Ketone. [On the effect of Caro’s reagent on ketones.] Ber. Dtsch. Chem. Ges., 33: 858-864. https://doi.org/10.1002/cber.190003301153