What is Criegee ozonolysis?
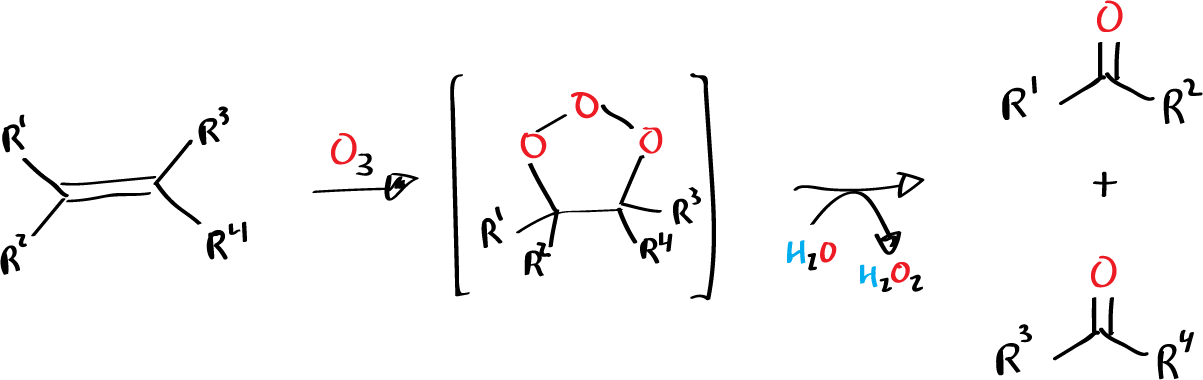
The Criegee ozonolysis is a chemical reaction in which an alkene is cleaved into two smaller molecules by the addition of ozone (O3) followed by the removal of the ozonide intermediate. The reaction proceeds through a syn-addition mechanism and produces aldehydes or ketones, depending on the alkene starting material.
In 1840, Christian Friedrich Schönbein discovered ozone and five years later, he conducted the first ozonolysis experiment. His report revealed that ethylene reacts with ozone resulting in no detectable odor of either substance. The ozonolysis process for alkenes is sometimes called Harries ozonolysis, as some credit Carl Dietrich Harries for its discovery. Although ozonolysis was discovered earlier, it was Criegee who delved into the mechanism of this reaction starting in the 1950s. As a result, Criegee is recognized as the pioneer of contemporary organic chemistry involving ozone, earning him the title of “father of modern ozone organic chemistry.” The chemical reaction between olefins and ozone leading to the formation of aldehydes, ketones, carboxylic acids, and other compounds is now commonly known as the Criegee ozonolysis or Criegee reaction.
Prior to modern spectroscopic techniques, ozonolysis played a vital role in determining the composition of organic molecules. Chemists would use ozonolysis to break down an unknown alkene into more easily identifiable fragments.
The mechanism of the Criegee ozonolysis involves the addition of ozone (O3) to the alkene in a syn-addition manner. The intermediate formed, called an ozonide, is then removed through a reduction or oxidation process. Depending on the alkene starting material, the products of the reaction will be either aldehydes or ketones..
Despite its many benefits, the Criegee ozonolysis does have some limitations. One of the biggest challenges is the need to handle ozone, which is a highly reactive and toxic gas. Additionally, the reaction is sensitive to impurities, which can lead to the formation of unwanted by-products..
Summary
Criegee ozonolysis is a powerful chemical reaction that has played a significant role in the development of modern organic synthesis. Its ability to cleave alkenes into smaller molecules, and its usefulness in the analysis of unknown compounds make it an essential tool in the field of organic chemistry..
Example
One example of the ozonolysis of an alkene is the reaction of cis-2-butene with ozone, which produces formaldehyde and formic acid:
cis-2-butene + ozone → formaldehyde + formic acid
In this reaction, the double bond in cis-2-butene is cleaved by ozone, yielding two carbonyl compounds, formaldehyde and formic acid.
Mechanism of reaction
The mechanism of ozonolysis of an alkene can be described in several steps:
- Step 1: Ozone (O3) attacks the alkene, forming a carbonyl oxide intermediate.
- Step 2: The carbonyl oxide intermediate then collapses, releasing ozone and forming a new alkoxy radical intermediate.
- Step 3: The alkoxy radical intermediate is then converted into a carbonyl compound by either reducing the carbonyl group or addition of a hydroxyl radical.
- Step 4: If the intermediate is reduced, it will yield an aldehyde or ketone. If it is attacked by a hydroxyl radical, it will yield a carboxylic acid or a formic acid.
- Step 5: the intermediate can also be attacked by another molecule of ozone to form a diol or a dialdehyde.
- Step 6: Depending on the structure and stereochemistry of the alkene, the ozonolysis can also produce different products.
References
- Schönbein, C. F. (1838–1840). “Lecture of 13 March 1839”. Ber. Verh. Nat. Ges. Basel. 4: 58
- Schönbein, C. F. (1840). “On the Odour Accompanying Electricity and on the Probability of its Dependence on the Presence of a New Substance”. Philosophical Magazine. 17: 293–294
- Schönbein, C. F. (1844). “On the Production of Ozone by Chemical Means”. Philosophical Magazine. 24: 466–467
- Harries, C. (1905), Ueber die Einwirkung des Ozons auf organische Verbindungen. [On the effect of ozone on organic compounds.] Justus Liebigs Ann. Chem., 343: 311-344. https://doi.org/10.1002/jlac.19053430209
- Staudinger, H. (1925), Über die Autoxydation organischer Verbindungen, V.: Über die Konstitution der Ozonide. [On the autoxidation of organic compounds, V.: On the constitution of ozonides.] Ber. dtsch. Chem. Ges. A/B, 58: 1088-1096. https://doi.org/10.1002/cber.19250580619
- Criegee, R. (1975), Mechanism of Ozonolysis. Angew. Chem. Int. Ed. Engl., 14: 745-752. https://doi.org/10.1002/anie.197507451