Written by J.A Dobado | Last Updated on May 2, 2024
What is Kuhn-Roth oxidation?
The Kuhn-Roth reaction, developed in 1933, is a method used for quantifying the number of methyl groups attached to the carbon atom in an organic molecule. This involves the oxidation of the organic compound with chromic acid H2CrO4 and sulfuric acid H2SO4, followed by steam distillation to collect the acetic acid CH3COOH produced.
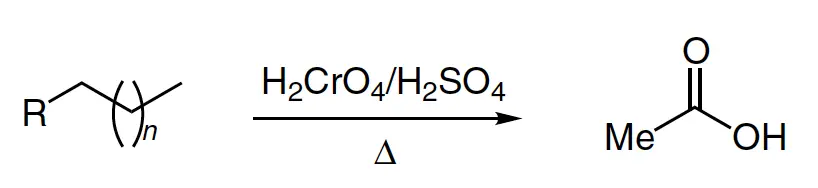
- R = alkyl, aryl (see list of acronyms)
- n > 1
The collected acetic acid can then be quantified via acid/base titration or converted to lithium acetate and further analyzed. The Kuhn-Roth oxidation is known by several names, including the Kuhn-Roth C-methyl determination, Kuhn-Roth chromic acid oxidation, and Kuhn-Roth degradation.
The ratio of chromic acid and sulfuric acid H2SO4 used in the reaction can vary depending on the nature of the organic molecules being analyzed. The collected acetic acid can be quantified using alkali hydroxide of varying concentrations, and phenolphthalein can be used as the endpoint indicator. In addition to methyl groups, this method can also measure the number of ethyl groups on cyclic compounds.
It’s important to note that the Kuhn-Roth oxidation only pertains to the oxidation at an extra-nuclear carbon atom and has a general reactivity order:
C6H5—CHR2 > R2CH > CH2R2 > RCH3 (R = aliphatic chain)
This method is accurate up to 20 carbon atoms on the side chains of monoalkylbenzenes but fails in the oxidation of toluene, ethylbenzene, and isopropylbenzene. For t-butylbenzene, the oxidation occurs exclusively at the ring.
Before the advent of modern spectroscopy, this method was widely utilized in the determination of the structure of natural products.
References
Kuhn, R. and Roth, H. (1933), Mikro-Bestimmung von Acetyl-, Benzoyl- und C-Methylgruppen. [Micro-determination of acetyl, benzoyl and C-methyl groups.] Ber. Dtsch. Chem. Ges. A/B, 66: 1274-1278. https://doi.org/10.1002/cber.19330660922