What is Pummerer rearrangement?
The transformation of a sulfoxide bearing an α-proton to α-substituted sulfide can be achieved through an acid or acyclic anhydride-promoted reaction, as reported by Pummerer in 1909. This reaction involves the formation of a sulfenium (or thionium) intermediate and can also result in the reduction of sulfonium sulfur with concomitant oxidation or substitution at the α-carbon of sulfonium. It is commonly referred to as the Pummerer rearrangement or Pummerer reaction, and is sometimes used to form heterocycles via the addition of an intramolecular nucleophile to the sulfenium intermediate.
However, when nucleophiles other than those derived from the anhydride are present, the reaction can result in different forms of functionalization.
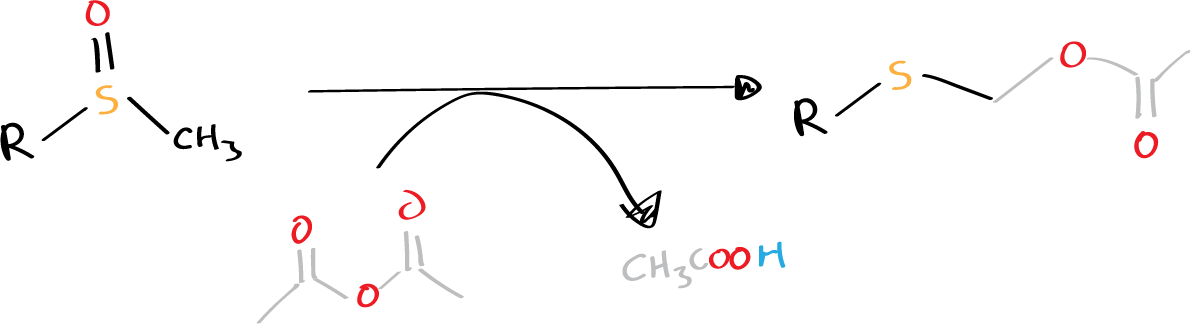
- R = alkyl, aryl (see list of acronyms)
Several promoters can be used to activate the Pummerer rearrangement, including HCl, acyclic anhydrides, p-toluenesulfonic acid, ZnCI2, TMSOTf, DAST (diethylaminosulfur trifluoride), and Me3SiX. In some cases, the reaction can occur under thermal conditions without promoters, although zeolite may be necessary to suppress it. The electrophilic nature of the sulfenium (or sulfonium) intermediate formed in this reaction allows it to react with even weak nucleophiles such as xylene and anisole.
References
- Pummerer, R. (1909), Über Brom-Additionsprodukte von Aryl-thioglykolsäuren. [On bromine addition products of aryl-thioglycolic acids.] Ber. Dtsch. Chem. Ges., 42: 2275-2282. https://doi.org/10.1002/cber.190904202125
- Pummerer, R. (1910), Über Phenylsulfoxy-essigsäure. (II.). [On phenylsulfoxyacetic acid. (II.).] Ber. Dtsch. Chem. Ges., 43: 1401-1412. https://doi.org/10.1002/cber.19100430241
Full Professor of Organic Chemistry at the University of Granada, with a long-standing research career in Computational Chemistry and molecular modeling and design.