What is Sabatier-Senderens reduction?
The origins of the hydrogenation reaction can be traced back to various discoveries, including Debus’s transformation of hydrocyanic acid into methylamine over platinum in 1863, De Wilde’s hydrogenation of acetylene into ethylene and ethane in 1874, and Mond’s extensive work from 1890 to 1895. However, it wasn’t until 1899 that Sabatier and Senderens established nickel-based hydrogenation, which involved passing the vapor of organic molecules and hydrogen over hot, finely divided nickel. This nickel-based vapor phase hydrogenation is now known as the Sabatier-Senderens reduction and is one of the most practically useful reactions, which earned Sabatier the Nobel Prize in 1912.
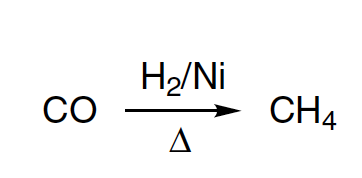
It’s worth noting that this reaction is distinct from reductions using nascent hydrogen as the reducing agent, such as amalgam of sodium in alcohol (alkaline condition) and or zinc or tin with hydrochloric acid (acidic medium). The success of the hydrogenation reaction depends on the purity of nickel and the reaction temperature, with trace amounts of sulfur, bromine, or iodine deactivating the nickel catalyst. Each hydrogenation process also takes place within a well-defined temperature range, as evidenced by the hydrogenation of benzene to cyclohexane at temperatures ranging from 70 to 190 ºC, with an optimal temperature between 170 and 190 ºC, and the further reduction of benzene to methane accompanied by the deposited carbon on nickel at temperatures > 300 ºC.
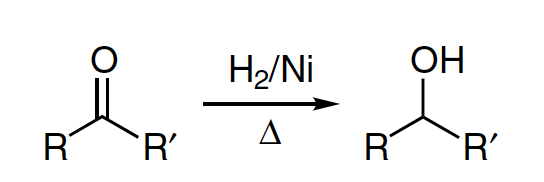
Under the correct hydrogenation conditions, Sabatier et al. were able to convert various organic molecules into their corresponding saturated compounds, such as oleic acid into stearic acid, acetone to isopropanol, carbon monoxide into methane or a gaseous mixture rich in methane, phenol and p-cresol into cyclohexanol and p-methylcyclohexanol, benzene to cyclohexane, and naphthalene to tetralin, among others. However, hydrogenation of naphthalene only yields tetralin, and other reducing methods may lead to unexpected products, as demonstrated by the reduction of benzene with hydroiodic acid at 250 ºC to afford methyl cyclopentane, and the hydrogenation of only one phenyl group of triphenylcarbinol over the Adams’ platinum oxide catalyst.
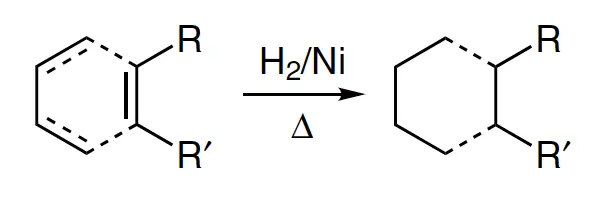
- R = alkyl
- R’ = H, alkyl (see list of acronyms)
Sabatier et al. also found that the reduced cobalt, iron, copper, and powdered platinum have catalytic activities similar to nickel, with copper being superior to nickel during the hydrogenation of nitrobenzene to aniline due to its insensitivity to accidental impurities. It’s worth noting that other researchers have successfully converted naphthalene into decalin over nickel, whereas Sabatier was only able to reduce it to tetralin.
References
- Sabatier, P. and Senderens, J. B., Compt. Rend., 1899, 128, 1173
- Sabatier, P. and Senderens, J. B., Compt. Rend., 1901, 132, 1254
- Sabatier, P. and Mailhe, A., Ann. Chim. Phys., 1908, 10, 527
- Mond, L.; Langer, C. and Quincke, F., J. Chem. Soc., 1890, 57, 749
- Mond, L.; Langer, C. and Quincke, F., Chem. News, 1890, 62, 97
- Mond, L. and Langer, C., J. Soc. Chem., Trans., 1891, 59, 1090
- Mond, L. and Quincke, F., J. Chem. Soc., Trans., 1891, 59, 604
- Mond, L., J. Soc. Chem. Ind., 1895, 14, 945