Written by J.A Dobado | Last Updated on May 2, 2024
What is Steglich esterification?
The Steglich catalyst, also known as 4-(N,N-dimethylamino)pyridine (DMAP), was first reported by Litvinenko and Kirichenko in 1967, and subsequently by Steglich in 1969.
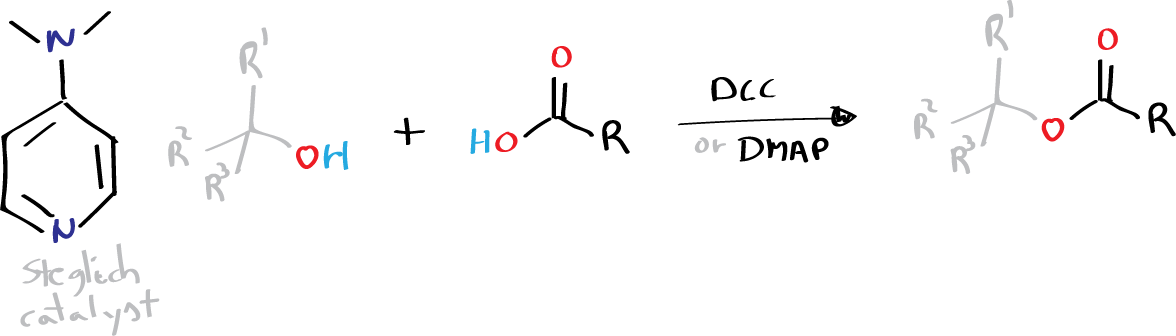
- R, R1, R2 = alkyl, aryl
- R3 = H, alkyl, aryl (see list of acronyms)
DMAP has been widely used as a super acylation catalyst for the preparation of esters from carboxylic acid, DCC, and secondary or tertiary alcohols. The ester preparation catalyzed by DMAP is known as the Steglich esterification, which usually requires only 10 mol % of DMAP in conjunction with at least one equivalent of tertiary base, or 1.1 equivalent of DCC when carboxylic acid is used directly.
DMAP has supercatalytic activity during acylation, which is clearly shown by the rate enhancement compared with the reactions carried out with other tertiary base catalysts. For example, the benzoylation of m-chloroaniline catalyzed by DMAP is about 106 times faster than the noncatalyzed reaction, indicating DMAP is more nucleophilic than aniline toward acyl chloride. DMAP has been used as an efficient catalyst in many important reactions, including acylation, silylation, tritylation, transesterification, polymerization, Darkin-West reaction, Baylis-Hillman reaction, the conversion of nitroalkane to nitrile oxide with BOC2O, and more importantly, the Steglich rearrangement.
Steglich esterification is a chemical reaction used to synthesize esters by reacting carboxylic acids with alcohols in the presence of a Steglich catalyst. The reaction was developed by Steglich in the 1970s and is named after him. It is an important method for the synthesis of esters, which are widely used as flavors, fragrances, and intermediates in the production of plastics, resins, and other materials..
The reaction mechanism of Steglich esterification involves the formation of an intermediate carboxylate ion, which is formed by the transfer of a proton from the carboxylic acid to a protonated alcohol molecule. The intermediate carboxylate ion then undergoes a deprotonation reaction to form the ester product..
One of the main advantages of Steglich esterification is its high yield and good selectivity. It is typically performed under mild reaction conditions, which makes it suitable for synthesizing sensitive and labile compounds..
References
- Litvinenko, L. M. and Kirichenko, A. I., Dokl. Chem. Engl. Transl., 1967, 763
- Litvinenko, L. M. and Kirichenko, A. I., Dokl. Akad. Nauk SSSR Ser. Chim., 1967, 176, 97
- Steglich, W. and Höfle, D., “4-Dimethylamino-pyridin, ein hochwirksamer Acylierungskatalysator” Angew. Chem., 1969, 81, 1001-1001. https://doi.org/10.1002/ange.19690812313
- Steglich, W. and Höfle, G. (1969), N,N-Dimethyl-4-pyridinamine, a Very Effective Acylation Catalyst. Angew. Chem. Int. Ed. Engl., 8: 981-981. https://doi.org/10.1002/anie.196909811