Written by J.A Dobado | Last Updated on May 2, 2024
What is Wagner-Meerwein rearrangement?
The Wagner-Meerwein rearrangement, also known as the Wagner-Meerwein migration or Wagner-Meerwein shift. This reaction is a class of 1,2-rearrangement of carbocation intermediates, from which the migratory group (vinyl, phenyl, alkyl, H, …) (see list of acronyms) rearranges from an adjacent carbon atom to the carbocation center to form a more stable carbocation or to alleviate the ring strain. It is named after the German chemists Alfred Wagner and Hans Meerwein, who first reported the reaction in 1899 and subsequently extended by Meerwein in 1914.
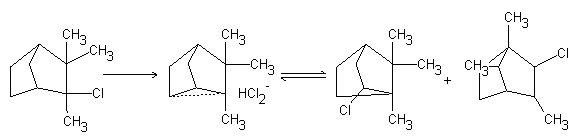
Wagner reported that the pinacol rearrangement and ring rearrangements of terpenes (such as, the dehydration of borneol and isoborneol to camphene) proceed through carbocations. Thus, Wagner described the idea that the acid-catalysed dehydration of borneol into camphene occurred via intra-molecular rearrangement. This rearrangement involves an intra-molecular 1,2-skeletal bond shift in the carbocation skeleton.
The Wagner–Meerwein rearrangement can be triggered by heat, light, or a chemical catalyst, such as acid or base. The reaction proceeds through a concerted mechanism, with the migration of the substituent occurring in a single step..
Also, the Wagner–Meerwein rearrangement has several key applications in organic chemistry. It is widely used in the synthesis of a variety of natural products and drugs. It has also been used in the synthesis of other heterocyclic compounds and in the preparation of polymers..
Summary
The Wagner–Meerwein rearrangement is an important reaction in the field of organic chemistry due to its ability to efficiently convert 1,2-disubstituted alkene into 1,3-disubstituted alkene, which have a wide range of applications in the synthesis of drugs and other organic compounds..
Example
An example of a Wagner-Meerwein rearrangement is the conversion of 2-butanol to 2-pentanol. The reaction can be catalyzed by acid or base catalysts, The mechanism of the reaction is thought to involve the formation of a carbocation intermediate, which then undergoes a 1,2-shift of the methyl group to form the product..
The balanced equation for the reaction is:
2-butanol + H+ → 2-pentanol + H2O
or
2-butanol + OH- → 2-pentanol + H2O
The reaction is thermally allowed due to the presence of a sigma and pi bond in the reactant, This is also known as a [1,2] sigmatropic shift..
It is important to note that the reaction rate is dependent on the stability of the carbocation intermediate which is formed. Carbocation intermediates with secondary and tertiary alkyl groups are more stable than primary alkyl group, which allows the rearrangement to occur more readily..
Mechanism of reaction
The mechanistic details of the Wagner-Meerwein rearrangement are still debated, but it is generally accepted that the reaction proceeds through a cyclic transition state in which the migrating alkyl group forms a covalent bond with the carbon atom to which it is migrating, while simultaneously breaking its bond with the carbon atom from which it is migrating.
In general, this reaction involves the formation of a cationic center, followed by the migration of a neighboring group to this carbocation center. The migrating group varies, depending on the migratory aptitude and the stereoelectronic effects.
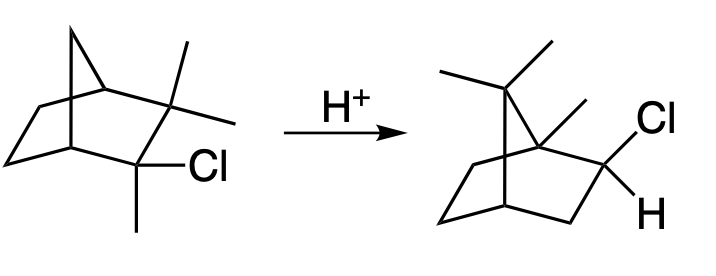
An example is given below for the rearrangement of camphene hydrochloride to isobornyl chloride.
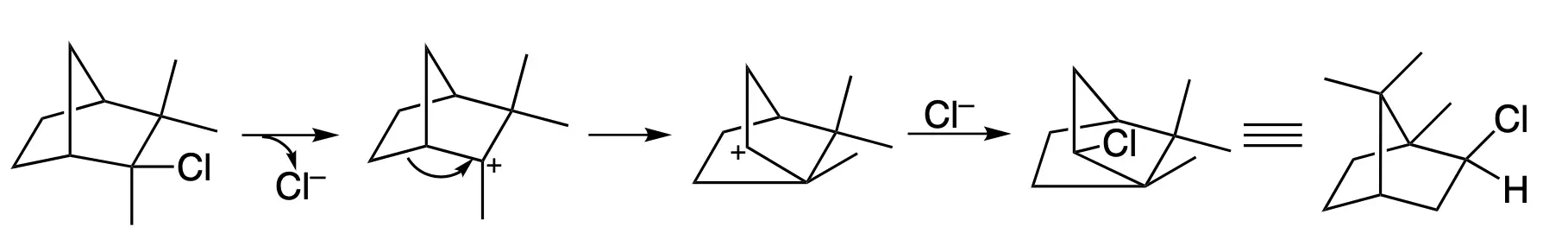
One proposed mechanism for the Wagner-Meerwein rearrangement is the “concerted” mechanism. This mechanism suggests that the migration of the alkyl group and the breaking of the original bond occur simultaneously in a single transition state. This is a concerted pericyclic reaction and is thermally allowed due to the presence of a sigma and pi bond in the reactant, This is also known as a [1,2] sigmatropic shift.
In addition, the migration of the alkyl group can also occur in a stepwise mechanism, where the migration and the breaking of the bond occur in two distinct steps.
For example, the mechanism of the Wagner-Meerwein rearrangement of 2-butanol to 2-pentanol can be explained in the following steps:
Step 1: Protonation of the alcohol by an acid catalyst (H+) or deprotonation by a base catalyst (OH-), forming a carbocation intermediate.
Step 2: Rearrangement of the carbocation intermediate by a 1,2-shift of the methyl group. This step involves the migration of the methyl group from the carbon atom at position 2 to position 3. The carbon-carbon bond between the carbon atom at position 2 and the carbon atom at position 3 is broken, and a new carbon-carbon bond is formed between the carbon atom at position 3 and the carbon atom that was originally bonded to the methyl group.
Step 3: Deprotonation by the base catalyst (OH-) or protonation by the acid catalyst (H+) to form the product, 2-pentanol.
The reaction is thermally allowed due to the presence of a sigma and pi bond in the reactant, This is also known as a [1,2] sigmatropic shift.
It is important to note that the stability of the carbocation intermediate is a crucial factor in determining the reaction rate. Secondary and tertiary carbocation are more stable than primary carbocation, which allows the rearrangement to occur more readily.
References
- Wagner, G., J. Russ. Phys. Chem. Soc., 1899, 31, 690.
- Meerwein, H. (1914), Über den Reaktionsmechanismus der Umwandlung von Borneol in Camphen; [Dritte Mitteilung über Pinakolinumlagerungen.]. [On the reaction mechanism of the conversion of borneol to camphene; [Third communication on pinacolin rearrangements.]] Justus Liebigs Ann. Chem., 405: 129-175. https://doi.org/10.1002/jlac.19144050202
- Meerwein, H. (1918), Über Ringveränderungen bei der Wasserabspaltung aus alicyclischen Alkoholen. [On ring changes in water splitting from alicyclic alcohols.] Justus Liebigs Ann. Chem., 417: 255-277. https://doi.org/10.1002/jlac.19184170205
- Meerwein, H. and van Emster, K. (1920), Unter-suchungen in der Camphen-Reihe, I.: Über den Reaktionsmechanismus der Isoborneol ⇌ Camphen-Umlagerung.[Investigations in the camphene series, I.: On the reaction mechanism of isoborneol ⇌ camphene rearrangement.] Ber. Dtsch. Chem. Ges. A/B, 53: 1815-1829. https://doi.org/10.1002/cber.19200530928
- Meerwein, H. and van Emster, K. (1922), Über die Gleichgewichts-Isomerie zwischen Bornylchlorid, Isobornylchlorid und Camphen-chlorhydrat.[On the equilibrium isomerism between bornyl chloride, isobornyl chloride and camphene chlorohydrate.] Ber. Dtsch. Chem. Ges. A/B, 55: 2500-2528. https://doi.org/10.1002/cber.19220550829
- Meerwein, H. and Schäfer, J. (1922), Über die wechselseitige Umwandlung von Verbindungen mit sechs- und siebengliedrigem Kohlenstoffring.[On the mutual transformation of compounds with six- and seven-membered carbon ring.] J. Prakt. Chem., 104: 289-310. https://doi.org/10.1002/prac.19221040121
- Meerwein, H. and Gérard, L. (1924), Untersuchungen über intramolekulare Atomverschiebungen. I. Über die Anlagerung von Alkoholen an Camphen. [Studies on intramolecular atomic displacements. I. On the addition of alcohols to camphene] Justus Liebigs Ann. Chem., 435: 174-189. https://doi.org/10.1002/jlac.19244350104
- Meerwein, H., Hammel, O., Serini, A. and Vorster, J. (1927), Untersuchungen über intramolekulare Atomverschiebungen in der Campherreihe.[Studies on intramolecular atomic displacements in the camphor series.] Justus Liebigs Ann. Chem., 453: 16-47. https://doi.org/10.1002/jlac.19274530103