Written by J.A Dobado | Last Updated on May 2, 2024
What is Zemplén deacetylation?
The Zemplén deacetylation is a reaction that was first reported by Zemplén and Kuntz in 1924. It is a useful method for the removal of O-acetyl protecting groups of carbohydrates, and it is performed by treating the O-acetylated substrates with a catalytic amount of sodium methoxide in methanol at room temperature. Zemplén deacetylation is also known as Zemplén de-O-acetylation, Zemplén deprotection, or Zemplén-deacetylation. It can also be referred to as Zemplén transesterification since the O-acetyl groups of carbohydrates are transferred onto methanol to form methyl acetate. Additionally, after the reaction, the O-acetyl-protected carbohydrates are transformed into unprotected ones, similar to the hydrolysis of an ester. Therefore, this reaction is sometimes referred to as Zemplén saponification.
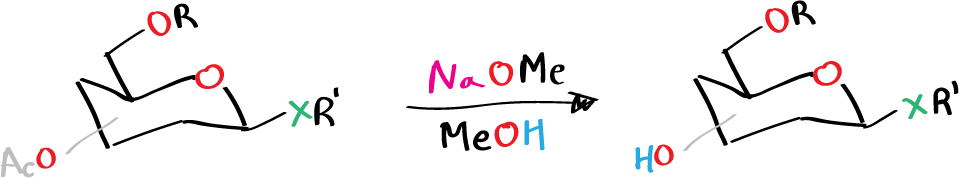
- R = alkyl, aryl, etc.
- X = NH, O, S, etc.
- R’ = alkyl, aryl, etc.
- R or R’ could be Ac and will be cleaved as well (see list of acronyms)
Compared to the regular hydrolysis of an ester, the Zemplén deacetylation requires only a catalytic amount of sodium methoxide instead of a stoichiometric amount of base for each ester functional group. Moreover, it usually gives an almost quantitative yield of carbohydrates. However, Zemplén deacetylation may not be suitable for the deprotection of carbohydrates with disulfide linkages due to the instability of the disulfide bond under basic conditions.
For instance, the deacetylation of (1S,5S,6S,7S,8R)-6,7,8-triacetoxy-9-oxa-2,3-dithiabicyclo[3.3.1]nonane in methanol with sodium methoxide or triethylamine affords only a small amount of (1S,5S,6S,7S,8R)-9-oxa-2,3-dithiabicyclo[3.3.1]nonane.
References
- Zemplén, G. and Kunz, A. (1924), Studien über Amygdalin, IV: Synthese des natürlichen l-Amygdalins. [Studies on amygdalin, IV: Synthesis of natural l-amygdalin.] Ber. Dtsch. Chem. Ges. A/B, 57: 1357-1359. https://doi.org/10.1002/cber.19240570825