Written by J.A Dobado | Last Updated on April 22, 2024
Objective
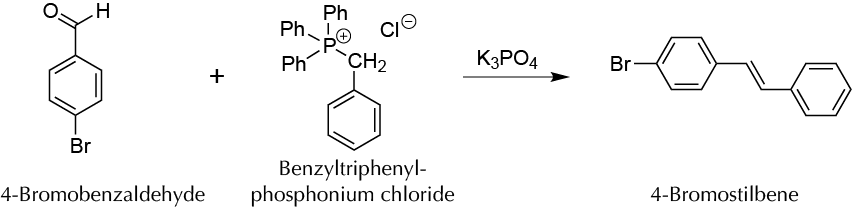
To conduct a Wittig reaction of carbon-carbon double bond C−C forming in the absence of solvent.
Background
The Wittig reaction is named after its discoverer Georg Wittig (Nobel Prize winner in 1979) and converts carbonyl compounds into alkenes. In such reactions, phosphorus ylides are produced from a phosphonium salt and a base. This ylide reacts with a carbonyl compound to give an alkene. Although, the Wittig reaction has a low atom economy, because triphenylphosphine oxide is formed as byproduct; however, this reaction can be improved in the absence of solvent.
Experimental procedure
In a porcelain mortar having an outer diameter of 80 mm, mix 200 mg of benzyltriphenylphosphonium chloride, 95 mg of 4-bromobenzaldehyde, and 425 mg of potassium phosphate. Grind the mixture in the mortar, doing circular movements with the pestle for 20 min. Stop the grinding periodically to gently scrape the inner surface of the mortar and the solid that remains stuck to the pestle with a spatula, to improve the mixing process. Resume grinding, so that the actual mixing time is at least 15 min, to be sure the reaction is complete. Check by TLC that the reaction has completed, using as eluent a mixture of ethyl acetate:hexane (1:4). To do this, take a few mg of the solid and dissolve in a few drops of EtOH, click the TLC plate with the capillary. Also, taking 1 mg of 4-bromobenzaldehyde dissolved in a few drops of EtOH, to use as a reference.
Add to the mortar 10 ml of deionized water and with a spatula scrape both sides of the mortar and pestle until it clears all solid and emulsion. The solid obtained is a mixture of E and Z isomers that are isolated by vacuum filtration. Drag the solid if necessary that has been glued to the wall of the mortar with 5 ml of extra water. The E isomer can be isolated as follows:
Transfer the solid to the isomer mixture to a test tube, add 2 ml of EtOH and heat in a water bath at 70-80 ºC until the solid is dissolved. Allow the solution to cool to room temperature Finally, cool the test tube in an ice bath until a solid corresponding to 4-bromoestilbene or (E)-(4-bromophenyl)-2-phenylethene appears (m.p. = 134-135 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| K3PO4 | 212.27 | - | - | 2.564 |
| Benzyltriphenylphosphonium chloride | 388.87 | 300 | - | - |
| 4-Bromostilbene | 259.14 | 138-142 | 341.99 | 1.372 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| EtOH |  |
| Ethyl acetate |   |
| Hexane |     |
| K3PO4 |  |
| Benzyltriphenylphosphonium chloride |  |
| 4-Bromostilbene | See MSDS |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| K3PO4 | LWIHDJKSTIGBAC-UHFFFAOYSA-K |
| Benzyltriphenylphosphonium chloride | USFRYJRPHFMVBZ-UHFFFAOYSA-M |
| 4-Bromostilbene | ZZMMKLVIBZWGPK-VOTSOKGWSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- S. H. Leung and S. A. Angel, Solvent-free Wittig reaction: a green organic chemistry laboratory experiment, Journal of Chemical Education 81 (2004), no. 10, 1492, DOI: 10.1021/ed081p1492