Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To reduce a cyclic ketone with sodium borohydride NaBH4 in an alkaline water solution.
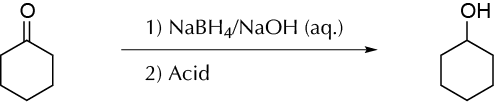
Background
Sodium borohydride (NaBH4) is a reagent that transforms aldehydes and ketones to the corresponding alcohol, primary or secondary, respectively. The conventional procedure is usually performed in MeOH or even with EtOH, but frequently the reaction with cyclohexanone as the starting product takes place with very poor yields. MeOH and EtOH react partially with NaBH4, producing hydrogen. NaBH4 is very soluble and relatively stable in aqueous alkali. For example, in a 0.2 M aqueous solution of NaOH, less than 5 % decomposition results in 2 weeks. The main limitation of water as a solvent is aldehydes and ketones is that most are insoluble in water. Cyclohexanone can be reduced easily in an aqueous medium, using a stirred solution of aqueous NaBH4 stabilized with sodium hydroxide. This procedure is not necessarily a general one, but it can be applied to carbonylic compounds, partially water soluble.
Experimental procedure
Prepare a solution of 30 ml of water and 5 drops of 10 % NaOH with a Pasteur pipette, in a 100 ml Erlenmeyer flask equipped with a stir bar. Add NaBH4 (1.5 g, 0.04 mol) to this solution with stirring. Place the flask in an ice-water bath, keeping the temperature below 40 ºC, throughout the process, and add dropwise cyclohexanone (9.8 g, 0.1 mol) while stirring, from a Pasteur pipette. After the addition is complete, stir the mixture for another 10 min at room temperature. Finally, dropwise slowly add 10 ml of HCl 6 M with stirring. Check the pH value to be sure that the solution is acidic, and add 10 g of NaCl and stir until the solution is saturated. The mixture is transferred to a funnel and extracted with diethylether (3 × 15 ml). Combine the organic extracts and dry in an appropriate flask with anhydrous sodium sulfate. Separate the drying agent by gravity filtration in a tared round-bottom flask. Fit a distillation apparatus to the flask and collect the fraction boiling at 15-65 ºC, such as cyclohexanol (estimated yield 70 %).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Cyclohexanol | 100.16 | 20-22 | 160-161 | 0.968 |
| Cyclohexanone | 98.14 | -47 | 155 | 0.947 |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaBH4 | 37.8 | 400 | - | 1.07 |
| NaCl | 58.44 | 801 | 1,413 | 2.165 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Cyclohexanol |  |
| Cyclohexanone |    |
| Diethyl ether |   |
| HCl |   |
| NaBH4 |    |
| NaCl | Non-hazardous |
| NaOH |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Cyclohexanol | HPXRVTGHNJAIIH-UHFFFAOYSA-N |
| Cyclohexanone | JHIVVAPYMSGYDF-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaBH4 | YOQDYZUWIQVZSF-UHFFFAOYSA-N |
| NaCl | FAPWRFPIFSIZLT-UHFFFAOYSA-M |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- N. J. Hudak and A. H. Sholes, Reduction of cyclohexanone with sodium borohydride in aqueous alkaline solution-a beginning organic chemistry experiment, Journal of Chemical Education 63 (1986), no. 2, 161, DOI: 10.1021/ed063p161
- N. M. Zaezek, Another look at reduction of cyclohexanone with sodium borohydride in aqueous alkaline solution, Journal of Chemical Education 63 (1986), no. 10, 909, DOI: 10.1021/ed063p909