Written by J.A Dobado | Last Updated on April 22, 2024
Objective
The goal of this experiment is the the reduction of the aromatic ketone benzophenone to diphenylmethanol (benzhydrol) using sodium borohydride, NaBH4, as a reducing agent.
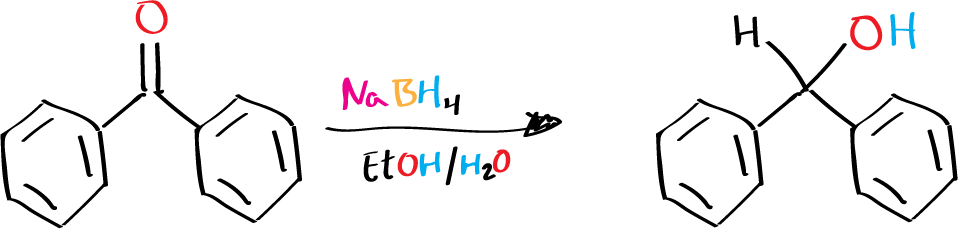
Background
The reduction of carbonyl groups is a fundamental reaction in organic chemistry. One commonly used reducing agent for this purpose is sodium borohydride (NaBH4), which is a mild and selective reducing agent that can reduce aldehydes, ketones, and other carbonyl-containing compounds. In this experiment, NaBH4 will be used to reduce the aromatic ketone benzophenone to diphenylmethanol (benzhydrol).
Benzophenone is a commonly used starting material in organic synthesis and is widely used as a photoinitiator in polymer chemistry. It is a highly reactive molecule that contains a carbonyl group, which makes it a suitable substrate for reduction by NaBH4. The reduction of benzophenone with NaBH4 proceeds via the transfer of a hydride ion from NaBH4 to the carbonyl carbon of benzophenone, resulting in the formation of an alcohol. The reaction is exothermic and is typically carried out in an organic solvent such as ethanol or aqueous ethanolic solution with an excess amount of the reducing agent to ensure complete reduction of the carbonyl group..
The reduction of benzophenone to diphenylmethanol is a useful laboratory experiment for students to gain experience with techniques such as crystallization, filtration, and thin-layer chromatography (TLC). The reaction is also ideal for working on a small scale and can be completed in a short period of time. The purity of the product can be assessed by TLC and its identity can be confirmed by melting point determination and infrared spectroscopy. Overall, this experiment provides a useful introduction to the reduction of carbonyl compounds and to the techniques of organic synthesis.
Experimental procedure
First, dissolve benzophenone in 5 mL of EtOH in a 25 mL Erlenmeyer flask, and stir the solution using a magnetic stirrer. In a separate small test-tube, dissolve NaBH4 in 1.5 mL of cold water, and add this solution one drop at a time to the stirred ethanolic solution of benzophenone at room temperature.
After adding all the NaBH4, continue to stir the mixture for another 40 minutes. Next, slowly pour the mixture into a 50 mL beaker containing a mixture of 10 mL of ice-water and 1 mL of concentrated hydrochloric acid, HCl. Wait a few minutes, then collect the precipitated product by vacuum filtration and wash it with two portions of 5 mL of water. Dry the crude product by suction at the filter pump for 10 minutes, then recrystallize it from the minimum volume of hot petroleum ether.
Record the yield and melting point of the product. Finally, compare the IR spectra of your diphenylmethanol and benzophenone to confirm the product’s identity.
Purity of the product
To check the purity of the recrystallized diphenylmethanol by TLC, dissolve ca. 10 mg of the product in a few drops of dichloromethane and spot this solution onto a silica gel TLC plate. Similarly, spot a reference solution of the starting ketone, benzophenone, onto the same plate. Develop the plate in petroleum ether-ethyl acetate (9:1). Visualize the developed plate under UV light and work out the Rf values for the two compounds.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Benzophenone | 182.22 | 47-51 | 305 | - |
| NaBH4 | 37.8 | 400 | - | 1.07 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Diphenylmethanol | 184.24 | 69 | 298 | 1.103 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| Petroleum ether | - | <-30 | 30-60 | 0.640 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Benzophenone |  |
| NaBH4 |    |
| EtOH |  |
| Diphenylmethanol |  |
| HCl |   |
| CH2Cl2 |  |
| Petroleum ether |     |
| Ethyl acetate |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Benzophenone | RWCCWEUUXYIKHB-UHFFFAOYSA-N |
| NaBH4 | YOQDYZUWIQVZSF-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Diphenylmethanol | QILSFLSDHQAZET-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| Petroleum ether | |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145