Written by J.A Dobado | Last Updated on April 22, 2024
Objective
To synthesize methyl diantilis using Merrifield, polymer-supported reagents from ethylvanillin. The use of polymer-supported reagents allows for efficient and convenient reactions with minimal by-products, which makes it an attractive option for organic synthesis.
Background
The preparation of methyl diantilis from ethylvanillin using Merrifield, polymer-supported reagents is a laboratory experiment that utilizes solid-phase peptide synthesis (SPPS) techniques to synthesize a diarylmethane derivative. This methodology is based on the use of Merrifield resin, a widely used solid support for the synthesis of peptides, as a polymer-supported reagent for organic synthesis. The method is attractive due to its high efficiency, reduced environmental impact, and the possibility of automation.
In this experiment, ethylvanillin, a vanillin derivative, is reacted with p-methoxybenzyl chloride in the presence of potassium carbonate to produce p-methoxybenzylethylvanillin. The p-methoxybenzyl group is then removed under mild acidic conditions, and the resulting intermediate is reacted with the resin-bound nucleophile, 4-(4′-methoxyphenyl)-2,4-dimethylpent-2-ene-4-ol, to form the diarylmethane product, methyl diantilis. The final product is purified by column chromatography and characterized by NMR spectroscopy.
This experiment provides students with an opportunity to learn and practice SPPS techniques, as well as gaining experience in organic synthesis and purification methods. It also allows for discussions on the advantages and disadvantages of using polymer-supported reagents in organic synthesis, and the importance of green chemistry principles in modern laboratory practices.
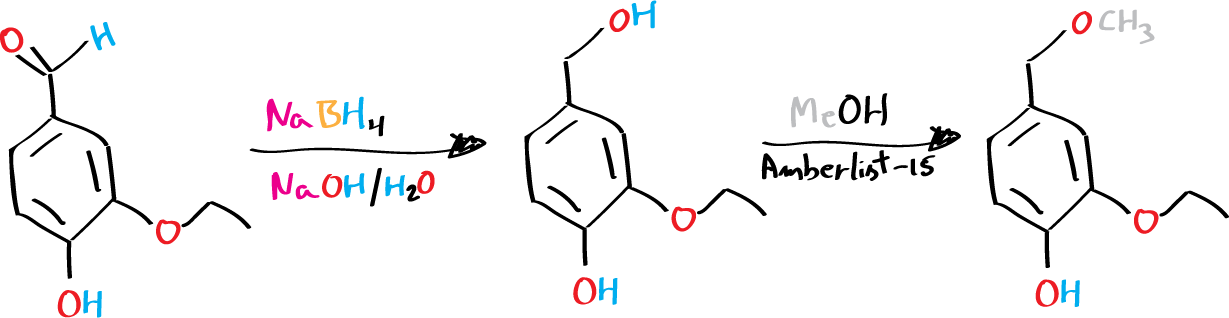
Experimental procedure
A) Preparation of 3‐ethoxy‐4‐hydroxybenzyl alcohol
In a 125 mL Erlenmeyer, with a stirbar, place the 3-ethoxy-4-hydroxybenzaldehyde and add 10 mL of 1.0 M NaOH solution. The flask should be cooled in an ice bath. Next, add 0.3 g of sodium borohydride, NaBH4 in small portions over 5 minutes while stirring the mixture.
After complete addition, allow the flask to warm to room temperature and stir the reaction mixture for a further 20 minutes. The reaction will change color from yellow to orange when it is complete. Then, cool the flask again in an ice bath and slowly add 7-8 mL of 2.0 M HCl until the pH of the solution is acidic. Leave the flask in the ice bath for 5 minutes after complete addition, then collect the precipitate by vacuum filtration. Wash the solid with 4×10 mL of water, then allow the solid to dry under suction for at least 15 minutes.
Record the yield and melting point of the product, and obtain the IR and 1H NMR spectra (in CDCl3) for further analysis.
B) Preparation of methyl diantilis
In a 25 mL round-bottom flask with a stirrer bar, add the ‘wet’ Amberlyst® 15. Wash the Amberlyst® 15 three times with 10 mL portions of MeOH, removing the MeOH by decanting it off the beads. After the final wash, add 15 mL of MeOH, and add 1.0 g of 3‐ethoxy‐4‐hydroxybenzyl alcohol, prepared in the previous step, in small portions under vigorous stirring.
Once the addition is complete, heat the reaction under reflux. After about 15 minutes, cool the reaction and monitor it by TLC, eluting with petroleum ether-ethyl acetate (1:1). Observe the developed plate under UV light and note the Rf values. If the reaction is incomplete, continue heating for another 15 minutes, then analyze by TLC again. For TLC analysis, remove 1 drop of the reaction mixture and dilute it with 5 drops of acetone.
After completion, allow the reaction to cool and add 0.5 g of sodium bicarbonate, NaHCO3, while continuing stirring for 5 minutes. Filter the mixture into another 25 mL round-bottom flask through gravity filtration. Rinse the residue with a few mL of MeOH, and then remove the solvent on the rotary evaporator.
Care must be taken not to overheat the product during the solvent removal process, as overheating can cause decomposition.
Although the product may not appear entirely dry, the presence of residual MeOH will not affect the interpretation of the 1H NMR spectrum.
Record the melting point, and collect the IR and 1H NMR spectra (CDCl3) of the product.
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Synthesis of Methyl Diantilis, a Commercially Important Fragrance
William H. Miles and Katelyn B. Connell
Journal of Chemical Education 2006 83 (2), 285
DOI: 10.1021/ed083p285