Objective
To synthesis of 7-hydroxy-3-carboxycoumarin in water by a tandem reaction (Knoevenagel condensation and Pinner reaction).
Background
Knoevenagel reaction is a condensation between an aldehyde or a ketone with an active hydrogen compound in the presence of a basic catalyst to yield α,β-unsaturated compounds. Usually the catalyst is a weakly basic amine, and the active hydrogen compound bears electron-withdrawing groups such as:
CO2R, COR, CHO, CN, or NO2 (see list of acronyms)
The reaction is usually performed in aprotic organic solvents such as DMF, MeCN, or pyridine, which play the double role of solvent and catalyst. Research on this carbon-carbon formation method continues to find new catalysts and reaction conditions according to Green Chemistry strategies such as microwave and ultrasound irradiation, solvent-free conditions, solid-phase synthesis, or use of water as an inexpensive and environmentally benign solvent.
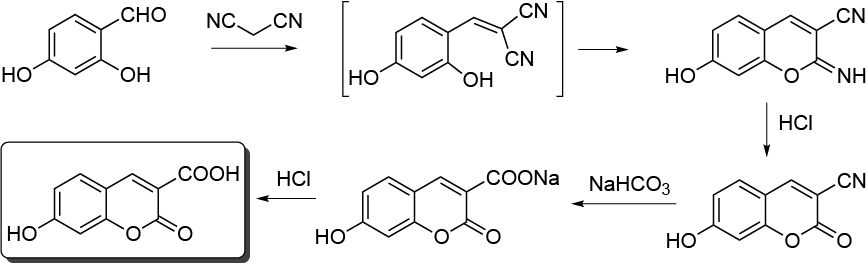
In this experiment, 7-hydroxy-3-carboxycoumarin is prepared by a one-pot consecutive process in water using starting materials 2,4-dihydroxybenzaldehyde and malononitrile. In the first step, a Knoevenagel condensation takes place, to produce an intermediate imine derivative that is hydrolyzed in situ to give the final product (Pinner reaction).
The procedure highlights the advantages of using water, with the possibility of controlling the pH and isolating the reaction product without using any organic solvent. The coumarin ring system is present in many natural products with useful pharmacological properties, for example, as an antitumoural, as anti-HIV agents, or as central nervous system effects.
Experimental procedure
Under a well-ventilated fume hood, vigorously stir finely powered 2,4-dihydroxy-benzaldehyde (1.38 g, 10 mmol), malononitrile (0.80 g, 12.5 mmol), and 0.05 M aqueous NaHCO3 solution (50 ml) for 1.5 h at r.t. in a 100 ml round-bottom flask fitted with a magnetic stir bar and reflux condenser. Then add concentrated HCl (1.25 ml), heat the heterogeneous mixture, and stir for 1 h at 90 ºC. After cooling the mixture, add 1 M aqueous NaHCO3 solution (20 ml) and heat the mixture again for 2 h at 90 ºC while stirring. The sodium salt of 7-hydroxy-3-carboxycoumarin is water soluble. Acidify the final solution, cooled to r.t., stir with concentrated HCl to pH = 2.0, and refrigerate at 0-5 ºC. Separate the precipitate 7-hydroxy-3 carboxycoumarin from the aqueous medium by vacuum filtration using a Büchner funnel and then dry (1.75 g, yield 85 %). The crude coumarin has a purity higher than 98 % and can be further purified by recrystallization from H2O/AcOH 8:2; m.p. = 248-250 ºC.
The process can be stopped after the Knoevenagel condensation and the Pinner reaction (1.5 h) or after the hydrolysis of the imino derivative (2.5 h) to produce 7-hydroxy-3-cyano-2-iminocoumarin (m.p. = 250 ºC from DMF/H2O 9:1) and 7-hydroxy-3-cyanocoumarin (m.p. = 273-275 ºC from H2O/AcOH 9:1), respectively, with high yield and purity (>95 %) by vacuum filtration of the aqueous phase. These can be suitable stopping points for two or three lab periods. In this case, the aqueous reaction mixture is stored at r.t. and the subsequent reactions carried out the next day.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Malononitrile | 66.06 | 30-32 | 220 | 1.049 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
| 2,4-Dihydroxybenzaldehyde | 138.12 | 135-137 | 220-228 | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| HCl |   |
| Malononitrile |   |
| NaHCO3 | Non-hazardous |
| 2,4-Dihydroxybenzaldehyde |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Malononitrile | CUONGYYJJVDODC-UHFFFAOYSA-N |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
| 2,4-Dihydroxybenzaldehyde | IUNJCFABHJZSKB-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- F. Fringuelli, O. Piermatti, and F. Pizzo, One-pot synthesis of 7-hydroxy-3- carboxycoumarin in water, Journal of Chemical Education 81 (2004), no. 6, 874–876, DOI: 10.1021/ed081p874