Written by J.A Dobado | Last Updated on April 22, 2024
Objective
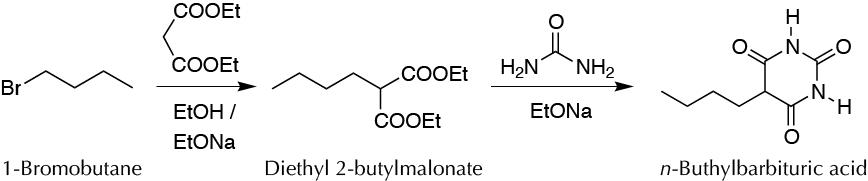
To perform a two-step synthesis for the preparation of a derivative of barbituric acid. In the first step, the diethyl n-butylmalonate is prepared, which is subsequently reacted with urea in the presence of a strong base, such as NaOEt, to yield the desired compound.
Background
Barbiturates are drugs that are prescribed to treat severe nervous insomnia, some forms of epilepsy, and certain convulsive and psychological disorders. Structurally, they are derived from barbituric acid. They are legal substances of controlled prescription with drug action and addictive effects, and thus prescription is required for their sale. Their use leads to tolerance, and suppression causes the stop-eating syndrome. Ever since von Baeyer (Nobel Prize in Chemistry 1905) synthesized barbituric acid in 1863, which has no sedative effects, over 2,500 derivatives of the substance have been investigated. For a long period of time, barbiturates and opiates were the only substances available to relieve anxiety or agitation of some psychiatric patients.
Experimental procedure
A) Preparation of diethyl n-butylmalonate:
In a 100 ml flask place absolute EtOH (50 ml) and sodium (1.2 g). Cut sodium into small pieces or, if possible, use sodium wire. Adapt a reflux condenser and fit a drying tube. Stir, at the beginning, at r.t. (the reaction generates heat due to a strongly exothermic character), and if necessary gently heat until the sodium disappears. Cool to r.t. and then add 8 g of diethyl n-malonate. Stir for about 15 min, and add 7.5 g of 1-bromobutane. Put the mixture to reflux for 1.5 h. After the reflux time, transfer the reaction crude to a separatory funnel, sliding the remaining crude with 30 ml of water. Stir the mixture vigorously, decanting and separating the aqueous layer. Extract the aqueous layer with diethyl ether (2 × 10 ml). Combine the organic extracts in an Erlenmeyer, and dry over anhydrous sodium sulfate. The desiccant is removed by gravity filtration. Next, remove the solvent under reduced pressure (rotary evaporator). Distill the residue under vacuum. Take into account the difference in b.p. of diethyl n-malonate (199.3 ºC) and of ethyl n-butylmalonate (234–240 ºC) at atmospheric pressure.
| DANGER! “Sodium reacts violently with water; therefore, all materials should be dry. At the beginning of the reaction, material projections and breaks in the flask may occur. Ensure that the ensemble (flask and reflux condenser) is firmly fixed with clamp and clamp holder.” |
B) Preparation of n-butylbarbituric acid:
Pour 50 ml of absolute EtOH into a dry 250 ml round-bottom flask. Then add 0.46 g of sodium and adjust a reflux condenser. Maintain the mixture at r.t. until the complete dissolution of sodium. Add the solution of NaOEt formed at 4.32 g of ethyl n-butylmalonate dry, followed by a solution of 1.2 g of urea predried in 22 ml of absolute EtOH. Reflux the mixture for 2 h. Then stop the reflux, allow the crude to cool to r.t., and gradually acidify the reaction mixture with 20 ml of 10% HCl (check the pH (acidity) with indicator paper). After neutralization of EtONa, remove EtOH under reduced pressure; a precipitate will appear. Complete the crystallization by placing the flask in an ice bath. Collect crystals by vacuum filtering, and wash with hexane to remove the unreacted diethyl n-butylmalonate (detected by smell). Recrystallize the solid from water using a proportion of approximately 20 ml per gram of product. Weigh the product dry and calculate the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| 1-Bromobutane | 137.02 | -112 | 100-104 | 1.276 |
| Barbituric acid | 128.09 | 248-252 | - | - |
| Diethyl ether | 74.12 | -116 | 34.6 | 0.71 |
| Diethyl malonate | 160.17 | -50 | 199 | 1.055 |
| Diethyl n-butylmalonate | 216.27 | - | 235-240 | 0.975 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Hexane | 86.18 | -95 | 69 | 0.659 |
| Na | 22.99 | 97.8 | 883 | 0.968 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| Urea | 60.06 | 132-135 | - | 1.335 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| 1-Bromobutane |    |
| Barbituric acid | Non-hazardous |
| Diethyl ether |   |
| Diethyl malonate |  |
| Diethyl n-butylmalonate |   |
| EtOH |  |
| HCl |   |
| Hexane |     |
| Na |   |
| Na2SO4 | Non-hazardous |
| Urea |  |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| 1-Bromobutane | MPPPKRYCTPRNTB-UHFFFAOYSA-N |
| Barbituric acid | HNYOPLTXPVRDBG-UHFFFAOYSA-N |
| Diethyl ether | RTZKZFJDLAIYFH-UHFFFAOYSA-N |
| Diethyl malonate | IYXGSMUGOJNHAZ-UHFFFAOYSA-N |
| Diethyl n-butylmalonate | RPNFNBGRHCUORR-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Hexane | VLKZOEOYAKHREP-UHFFFAOYSA-N |
| Na | KEAYESYHFKHZAL-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| Urea | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145