Written by J.A Dobado | Last Updated on April 22, 2024
What is 2-methyl-2-propanol?
The 2-methyl-2-propanol or terbutanol (t-BuOH) is a tertiary alcohol (the simplest of its homologous series). Its molecular formula is (CH3)3COH and molecular mass of 74.123 g-mol−1, it is also known as trimethyl carbinol. It is one of the four isomers of butanol. Its IUPAC name it is the 2-methylpropan-2-ol.
2-methyl-2-propanol, also known as tert-butanol or 2-methylpropane-2-ol, is a clear, colorless liquid with a sweet, fruity odor. It is a structural isomer of 1-propanol, with the methyl groups attached to the second and third carbons rather than the first.
2-methyl-2-propanol is used as a solvent in the production of paints, varnishes, and other coatings. It is also used as an intermediate in the synthesis of other chemicals, such as 2-methylpropan-1,3-diol and 2-methylpropan-2-yl acetate. In addition, it is used as a fuel additive to improve the octane rating of gasoline and to reduce emissions.
Applications
It is used as a solvent, in denatured ethanol, as an ingredient in paint cleaners and as a chemical intermediate to produce methyl tertiary butyl ether (MTBE) and ethyl tertiary butyl ether (ETBE) by reaction with methanol and ethanol, respectively. It is also used to obtain tertiary butyl hydroperoxide (TBHP) by reaction with hydrogen peroxide (see list of acronyms).
Overall, 2-methyl-2-propanol is a useful chemical with a wide range of applications in the chemical and fuel industries. Its unique chemical structure and properties make it an important molecule to consider in the synthesis and formulation of various products.
Chemical structure
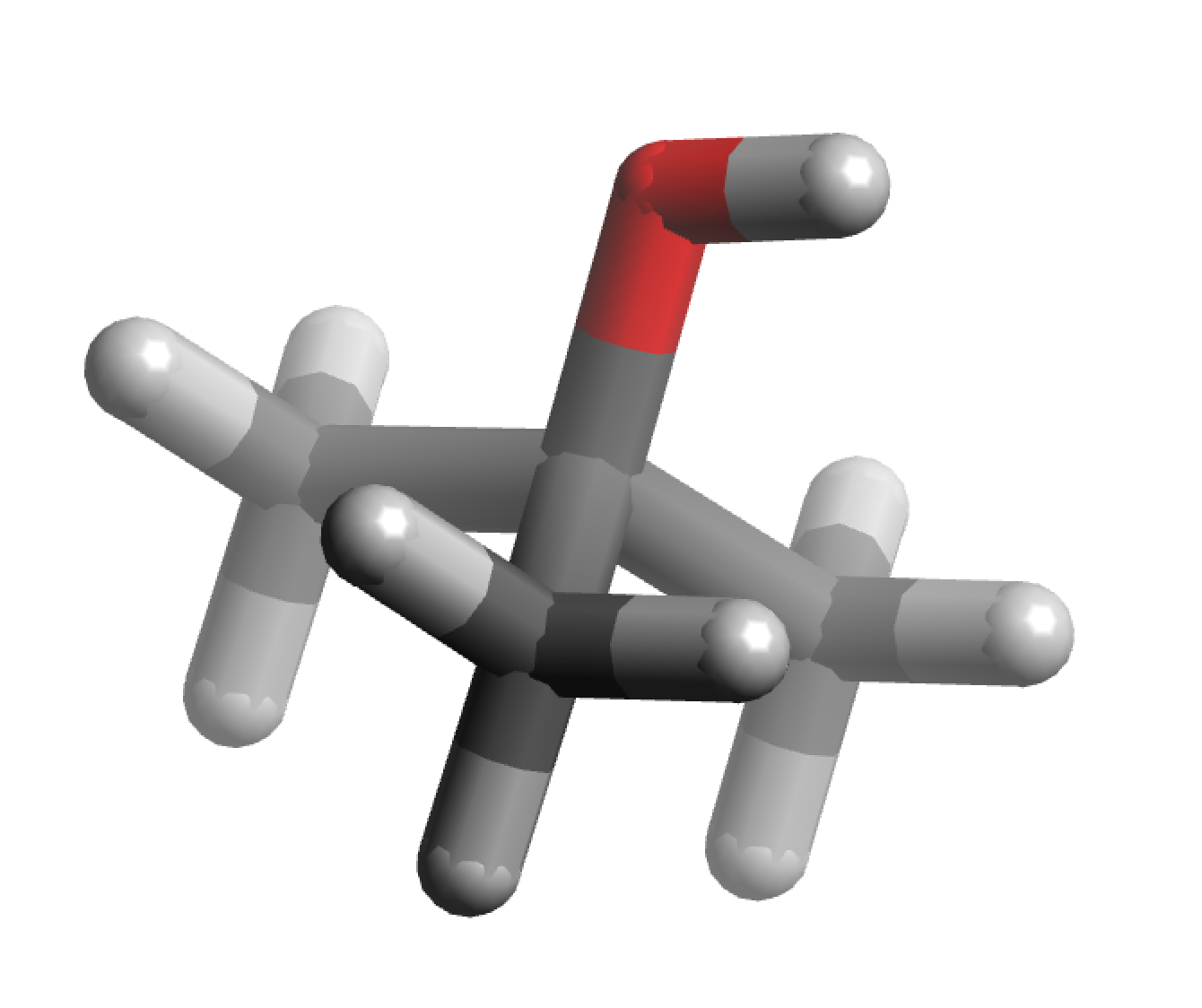 |
| 3D structure |
It is one of the four isomers (with the same molecular formula C4H10O) of butanol. The other three are isobutanol (CH3)2CHCH2OH, 2-butanol CH3CH2CH(OH)CH3, and n-butanol CH3CH2CH2CH2OH. It has a dipole moment of 1.31 Debye.
Physico-chemical properties
The 2-methyl-2-propanol is a colorless solid, which melts at near room temperature (25-26 ºC) and has a camphor-like odor. It is miscible in water, ethanol and diethyl ether. At room temperature, 2-methyl-2-propanol is a liquid with a boiling point is 82-83 ºC and its density is 0.775 g-mL−1 . It is flammable and should be handled with caution. Inhaling large amounts of the vapor can be harmful, and contact with the skin and eyes should be avoided.
Like all tertiary alcohols, it is more difficult to oxidize than the other butanol isomers.
Terbutanol is deprotonated with a strong base such as an alkoxide, e.g. potassium terbutoxide, which is prepared by treatment of terbutanol with potassium metal.
- K + t-BuOH → t-BuO−K+ + 1/2 H2
Thus, the terbutoxide obtained is a strong, non-nucleophilic base widely used in Organic Synthesis. It has the ability to remove acidic protons (H+) from substrates, and its steric hindrance prevents it from participating in nucleophilic substitutions, such as the Williamson ether synthesis or an SN2 nucleophilic substitution reaction.
On the other hand, terbutanol reacts with HCl to yield tert-butyl chloride.
Chlorination in oxygen (O-chlorination) of terbutanol with hypochlorous acid (HOCl) gives tert-butyl hypochlorite according to the following reaction:
(CH3)3COH + HOCl → (CH3)3COCl + H2O
Method of production
Tert-butanol is obtained commercially from isobutane as a by-product of propylene oxide production. It can also be produced by catalytic hydration of isobutylene, or by a Grignard reaction between methyl magnesium chloride and acetone.
Other reactions for productions include the hydroformylation of isobutylene, followed by hydrogenation of the resulting aldehyde. It can also be synthesized from acetone via the Meerwein-Ponndorf-Verley reduction or the Baeyer-Villiger oxidation.
Purification (drying)
Because it forms an azeotrope with water, its purification cannot be carried out by simple distillation. An initial drying (when it contains large amounts of water) can be done by adding benzene (tertiary azeotrope terbutanol/water/benzene) and removing the water by distillation.
If small amounts of water are present in the terbutanol, they can be removed by drying with an anhydrous salt such as calcium oxide (CaO), potassium carbonate (K2CO3), calcium sulfate (CaSO4) or magnesium sulfate (MgSO4), followed by fractional distillation. In a next step, anhydrous terbutanol can be obtained by refluxing and distilling from iodine-activated magnesium or alkali metals such as sodium and potassium.
Other methods used to obtain anhydrous terbutanol are 4 Å molecular sieves, aluminium terbutylation or calcium hydride (CaH2).