Written by J.A Dobado | Last Updated on April 22, 2024
What are alkynes?
Alkynes are compounds that have at least one C≡C triple bond. When there is only one triple bond in the molecule its general formula is CnH2n-2.
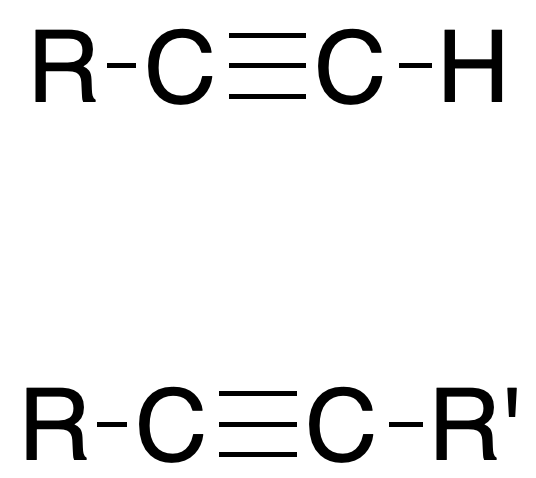
Formulation of alkynes
Follow the link for a summary of the formulation and nomenclature rules for alkynes.
Reactions of alkyne
When studying the reactivity of alkynes (C≡C) two possibilities have to be considered depending on whether it is a terminal alkyne or a disubstituted alkyne, because although they share reactivity, the terminals have some special reactions that the disubstituted ones do not give. These reactions are detailed in the section on alkynes reactions.
Analysis of alkynes
Like alkenes, alkynes are soluble in concentrated H2SO4, add bromine in CCl4 and are oxidized by cold aqueous permanganate.
If the alkyne is terminal, it will exhibit, in the IR spectrum, a characteristic sharp band in the range 3310-3200 cm-1. The triple bond stress band appears with an average intensity of 2140 to 2100 cm-1. If it is not terminal the triple bond band appears in the range 2250-2150 cm-1.
Alkaline mercuric iodide test
This test is applicable only to terminal alkynes.
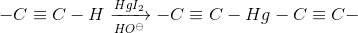
Procedure : Prepare Nessler’s reagent by dissolving 6.6 g of mercuric chloride HgI2 in a solution of 16.3 g of potassium iodide KI in 16.3 ml of water and then add 125 ml of 10 % NaOH. We let cool and dissolve the alkyne in 20 times its volume of EtOH. To add drop by drop this dissolution on 2 ml of the previous reagent.
The appearance of a white or grayish precipitate indicates the presence of a terminal alkyne.
If we shake for 2 or 3 min, filter and wash with EtOH at 50 % we would obtain the mercuric derivative, whose handling must be done with care since many of them are explosive when dry.