Written by J.A Dobado | Last Updated on April 22, 2024
What are amines?
Amines are compounds that have the functional group -NH2.
They are usually classified according to the number of hydrogen atoms in the ammonia (NH3) that are substituted by other groups into primary (one substituent), secondary (two), tertiary (three) and quaternary (four substituents and a positive charge is added).
Acid-base properties
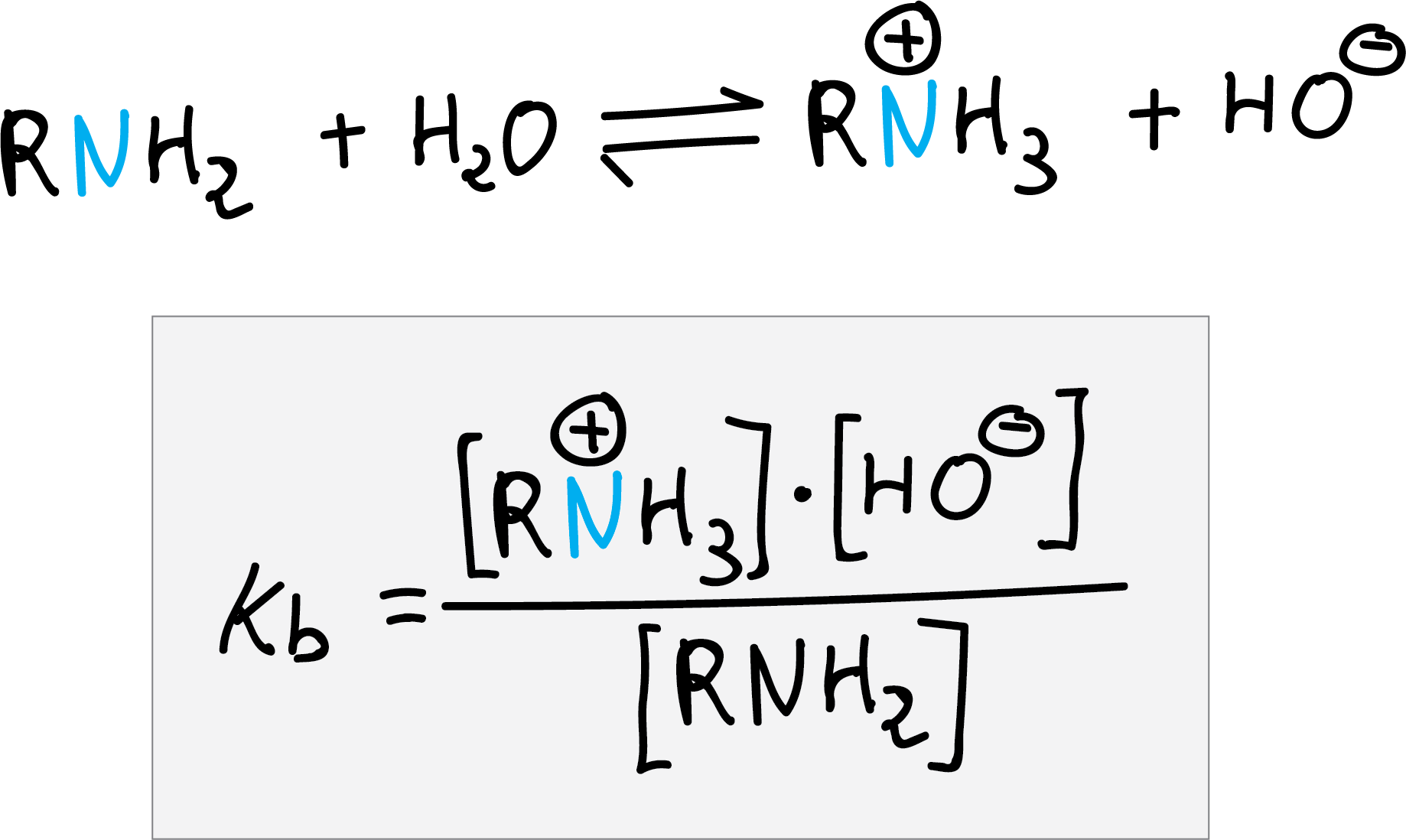
Amines are more basic than water but less than the hydroxide ion, the equilibrium constant for the reaction with water is called the basicity constant (Kb).
The values of (Kb) for aliphatic amines range from 0.001 to 0.0001 (see Appendix C).
Aromatic amines are usually less basic than aliphatic amines. The pKa or pKb values of the conjugate acid or base are obtained from the following relationship: pKa + pKb = 14.
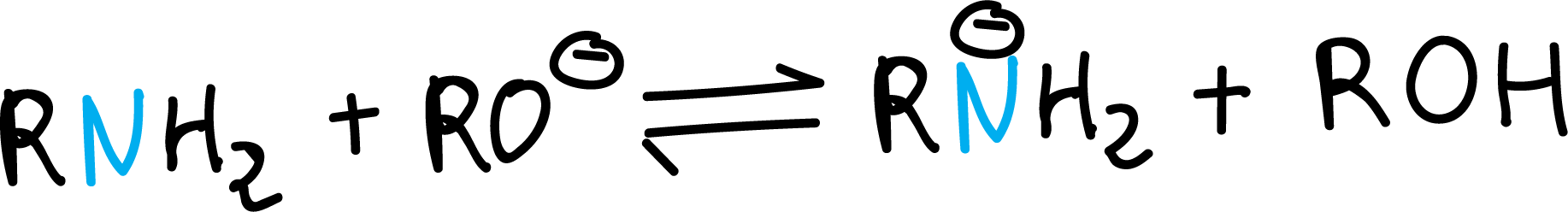
In addition, with much stronger bases such as alkoxides, they behave like acids, losing a proton to give the corresponding amide.
Nomenclature
Follow the link for a summary of amine nomenclature rules.
Reactions
The reactivity of amines is usually studied by separating them into two groups: on the one hand the reactions of aliphatic amines and on the other hand aromatic amines (arylamines).
Analysis of amines
Most simple amines are easily recognized by their solubility in dilute mineral acids. Water-soluble amines can be determined by their basic reaction using litmus or other indicators.
Primary amines, both aliphatic and aromatic, show, in the IR spectrum, a weak but recognizable doublet in the region from 3500 to 3300 cm-1 and a strong absorption in the region from 1640 to 1560 cm-1.
Secondary amines show an isolated band in the region of 3450 to 3310 cm-1. Tertiary amines do not show characteristic useful absorptions.
Copper (Cu2+) ion test
Procedure: Add 10 mg or a small drop of the compound over 0.5 ml of a 10 % solution of cupric sulfate.
The appearance of a blue or blue-greenish coloration or precipitate is indicative of the presence of an amine.
The Hinsberg test
Suspend the suspected amine in NaOH solution. By adding benzenesulfonyl chloride:
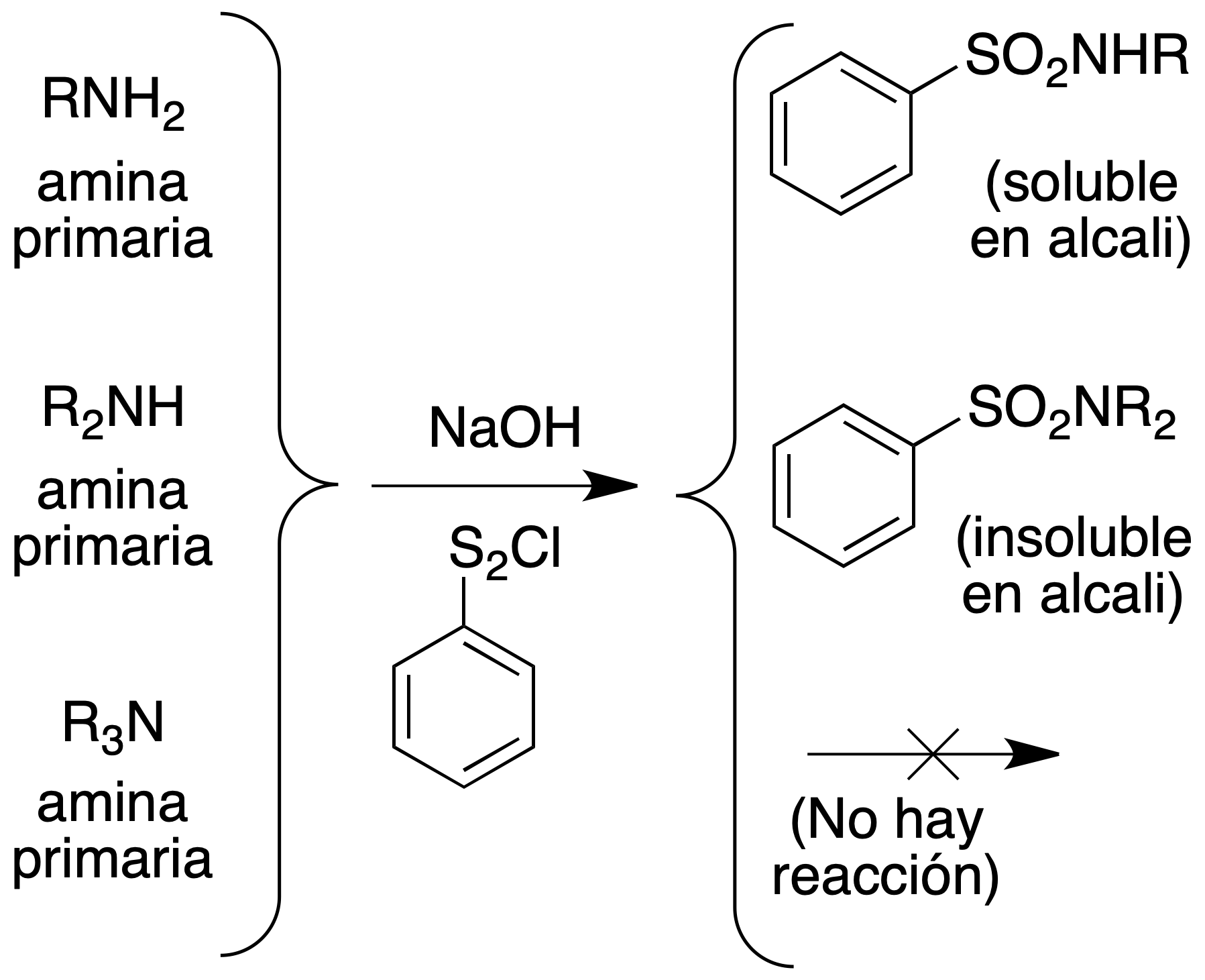
- The primary amines form sulfonamides which remain dissolved in the strongly alkaline solution. Upon acidification, the white solid sulfonamide precipitates.
- Secondary amines form sulfonamides which do not remain in solution but precipitate directly as white solids from the alkaline reaction mixture, insoluble in water, alkalis and dilute acids.
- Tertiary amines do not react, remain undissolved and dissolve upon acidification.
Procedure: 0.1 ml or 100 mg of the amine, 200 mg of p-toluensulfonyl chloride and 5 ml of 10 % NaOH solution are placed in a test tube. The tube is capped and shaken for 5 min.
Remove the stopper and heat 1 min. If no reaction is produced the substance is a tertiary amine, probably. If a precipitate appears dilute with 5 ml of water and shake.
If it does not dissolve it is probably a secondary amine. If it dissolves, acidify with dilute HCl. If the precipitate appears again, it is a primary amine.
Nitrous acid test (β-naphthol test) for aromatic amines
Procedure: Dissolve 100 mg of amiaminene in 3 ml of 2 N (or 5 %) HCl and cool it in an ice bath. Add 1 ml of 10 % solution of sodium nitrite in water.
If nitrogen release occurs, it is indicative of primary aliphatic amine. If on gentle heating nitrogen release occurs, it is indicative of primary amine.
If an aromatic primary amine is involved the diazonium salt can be trapped with a solution of 100 mg of β-naphthol in 2 ml of 10 % NaOH. Added dropwise over the above solution gives a red precipitate of the corresponding azo dye. The dye can also be orange.
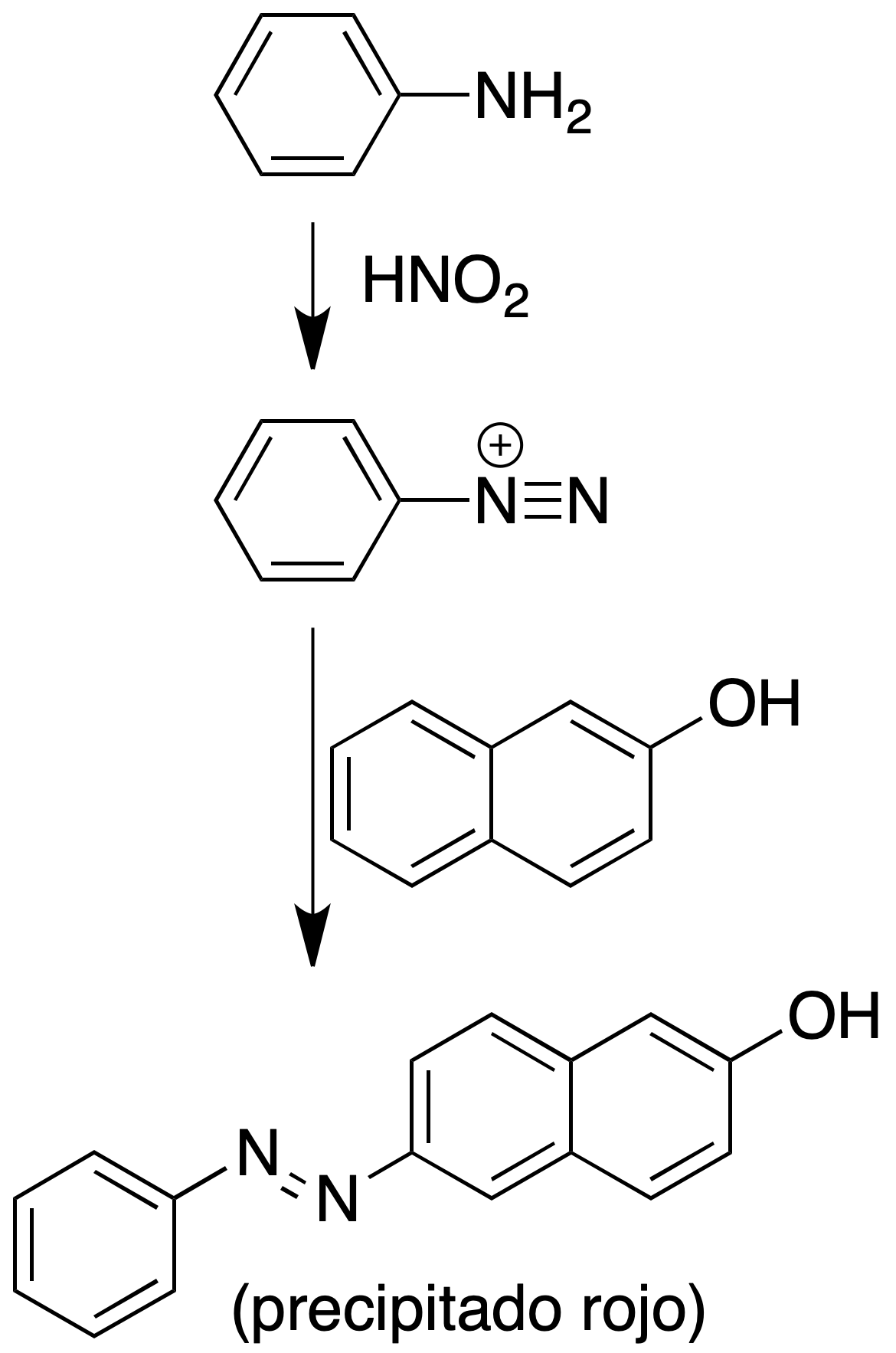
If an insoluble yellow compound (oil or solid) is formed during nitrite treatment, it is a secondary amine.
If no reaction is observed, it is an aliphatic tertiary amine.
If it is an aromatic tertiary amine, the p-nitrosated derivative can be formed (if the position is free), recognizable as a yellow compound, soluble in water and giving a greenish precipitate under the action of alkalis.
Amine formation
Amines can be characterized by the following reactions:
Sulfonamide formation
Procedure: A mixture of 150 mg of sulfonyl chloride (benzene or p-toluensulfonyl) and 200 mg of the amine is refluxed for 5-10 min in 4 ml of dry benzene. The mixture is allowed to cool. The precipitated amine hydrochloride is removed by filtration. From the filtrate the benzene is evaporated to obtain the crude sulfonamide, which can be recrystallized from EtOH.
Benzamide formation
Procedure: Approximately 150 mg of the amine is suspended in 1 ml of 10 % NaOH and 0.5 ml of benzoyl chloride is added dropwise while stirring vigorously and cooling.
After about 10 min it is carefully neutralized to pH = 8. The benzamide is filtered, washed with water and recrystallized from EtOH/H2O.
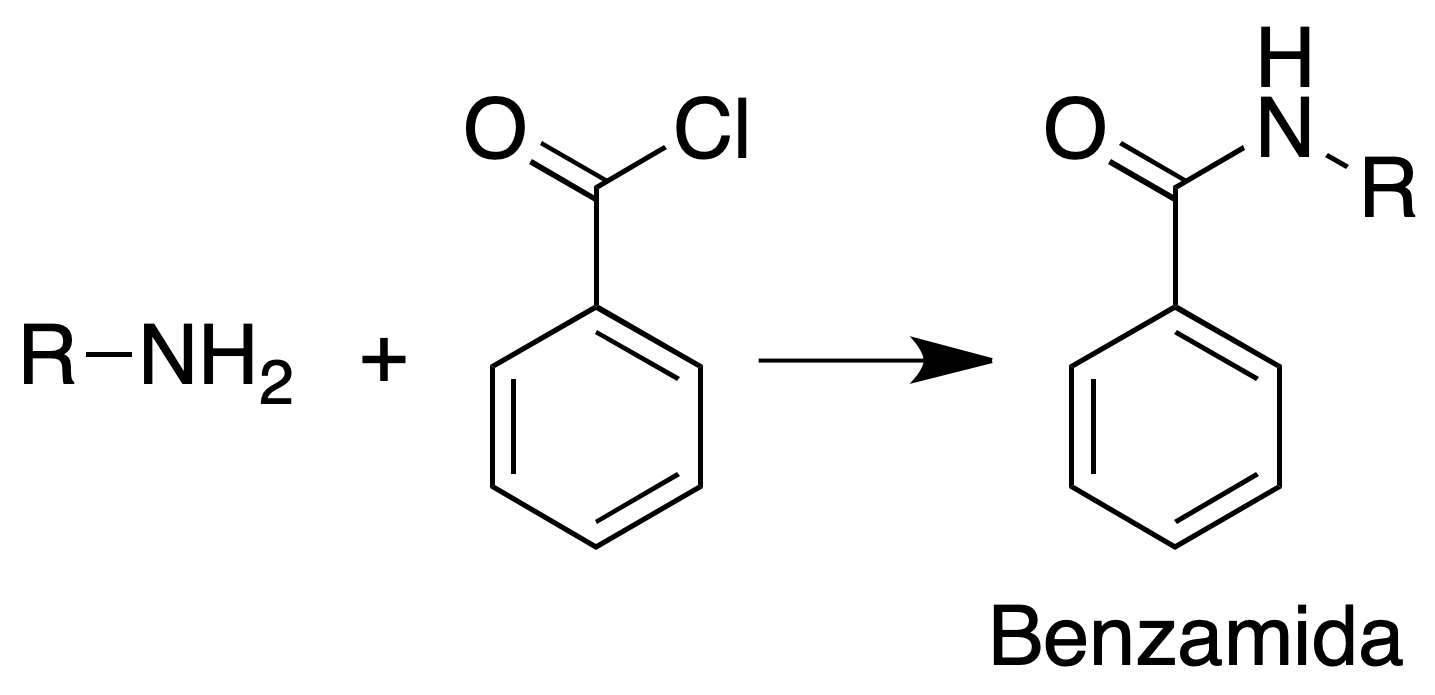
An alternative method consists in heating the amine and the benzoyl chloride dissolved in 2 ml of pyridine to reflux for 30 min. It is poured over ice water and the derivative is collected by filtration.
Acetamide formation
Procedure: Dissolve about 200 mg of water insoluble amine in 10 ml of 5 % HCl. Add 5 % NaOH with a burette until incipient turbidity.
To eliminate the turbidity with a few drops of 5% HCl. Add a little ice and 1 ml of acetic anhydride.
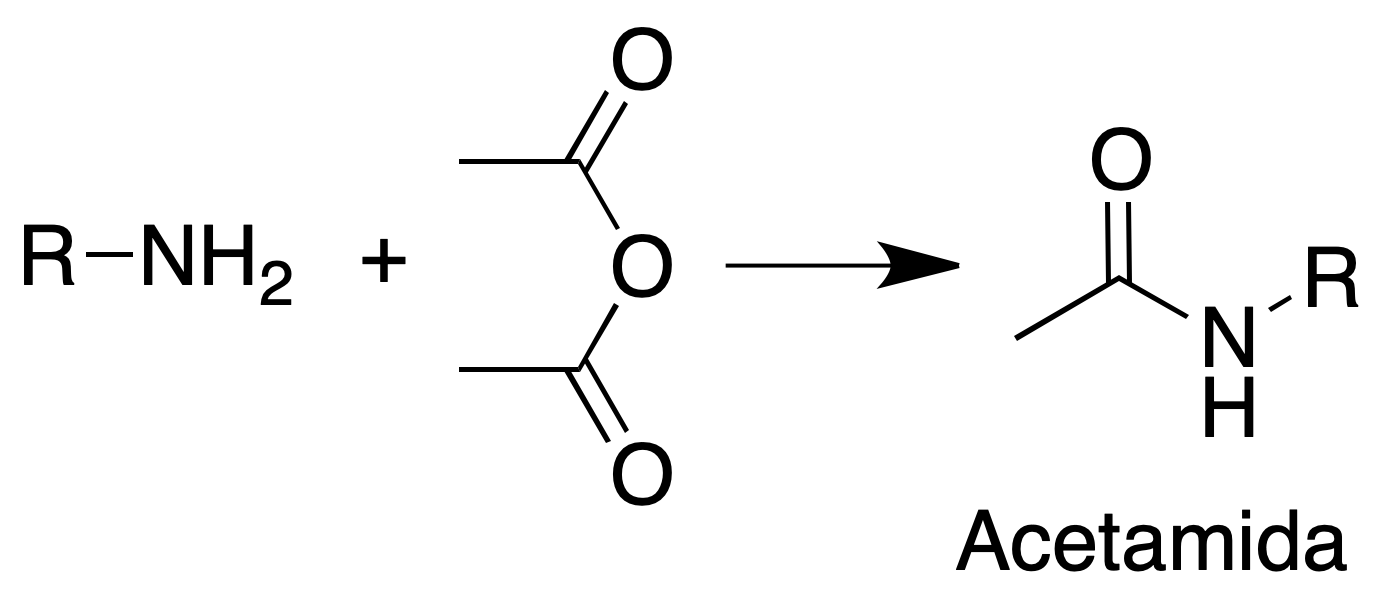
Stir the mixture and add 1 g of hydrated sodium acetate dissolved in 2 ml of water.
Cool in an ice water bath and filter the solid. Recrystallize from EtOH/H2O.
Picrate formation
They are mainly used as derivatives of tertiary amines.
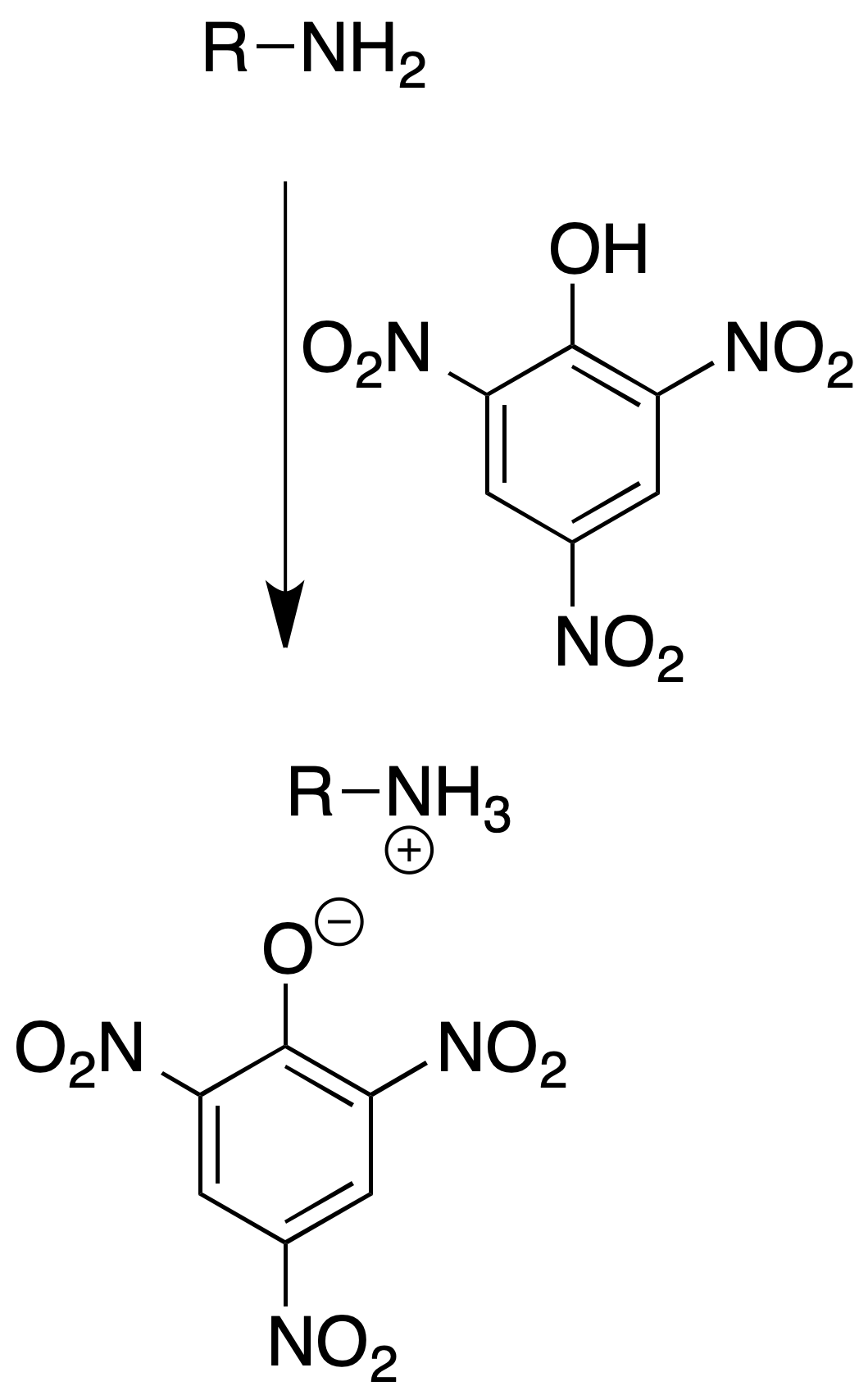
Procedure: Dissolve about 100 mg of amine in 5 ml of EtOH. Add 5 ml of saturated solution of picric acid in EtOH.
Heat the solution to boiling and allow to cool slowly. The formed yellow crystals can be recrystallized from MeOH or EtOH.